Question
HLPaper 1A
Ethylene glycol is used as an antifreeze chemical. If a person ingests it accidentally, ethylene glycol is rapidly converted by a series of enzyme-catalysed reactions in the liver to oxalic acid, which is toxic. The diagram summarizes the steps and enzymes involved in the conversion of ethylene glycol to oxalic acid.
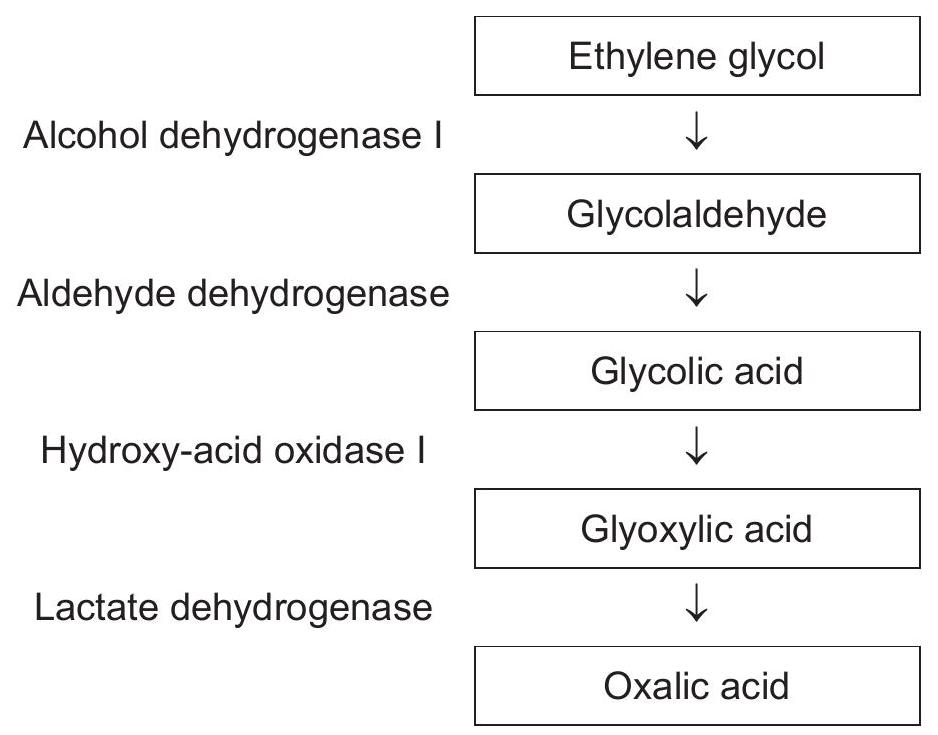
The production of oxalic acid can be prevented if the person drinks ethanol, a competitive inhibitor of the enzyme alcohol dehydrogenase I. Which statement explains the mode of action of ethanol on the reaction?

