Bromine and methanoic acid react in aqueous solution.
Br2 (aq) + HCOOH (aq) → 2Br− (aq) + 2H+ (aq) + CO2 (g)
The reaction was monitored by measuring the volume of carbon dioxide produced as time
progressed.
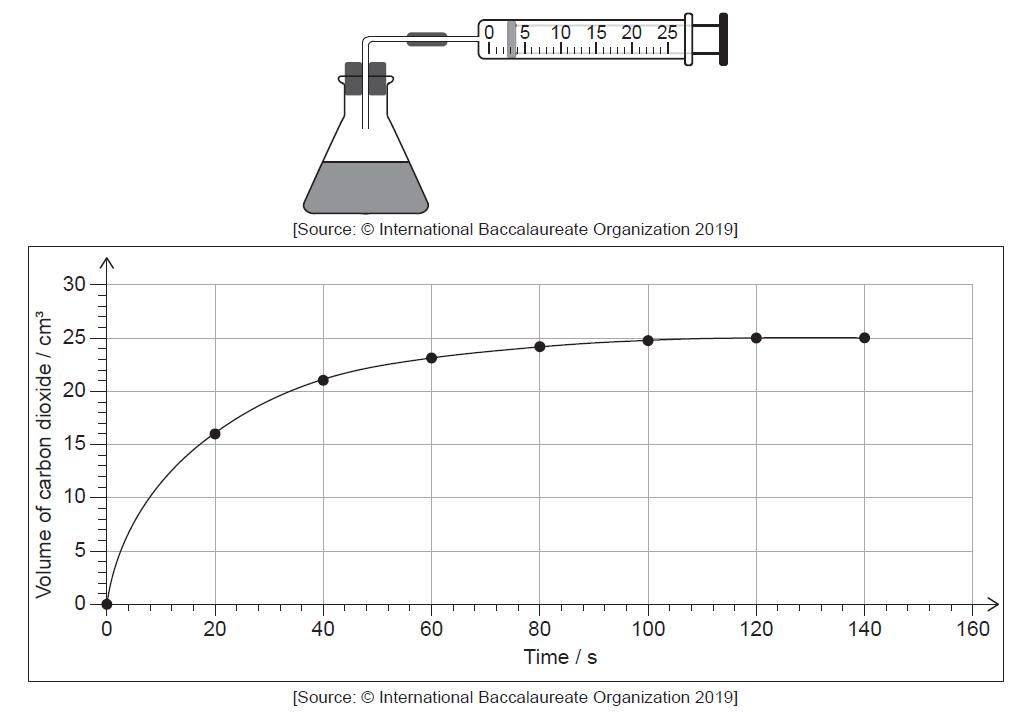
Determine from the graph the rate of reaction at 20 s, in cm3 s−1, showing your working.
tangent drawn to curve at t = 20 s
slope/gradient calculation
0.35 «cm3 s–1»
**Note:**Accept values in the range 0.32–0.42«cm3 s–1»
Outline, with a reason, another property that could be monitored to measure the rate of this reaction.
ALTERNATIVE 1
colour
Br2 /reactant is coloured «Br– (aq) is not»
ALTERNATIVE 2
conductivity****
greater/increased concentration of ions in products****
**Note:**Do not accept “changes in temperature” or “number of bubbles”.
ALTERNATIVE 3
mass/pressure
gas is evolved/produced
**Note:**Do not accept “mass of products is less than mass of reactants”.
ALTERNATIVE 4
pH
methanoic acid is weak AND HBr is strong
OR
increase in
Describe one systematic error associated with the use of the gas syringe, and how the error affects the calculated rate.
c(i).
ALTERNATIVE 1
gas may leak/be lost/escape
OR
plunger may stick/friction «so pressure is greater than atmospheric pressure»
OR
syringe may be tilted «up» so plunger moves less «with gravity acting on plunger»
OR
CO2 dissolved in water
calculated rate lower
ALTERNATIVE 2
syringe may be tilted «down» so plunger moves more «with gravity acting on plunger»
OR
syringe is held in hand so gets warmer and gas expands****
calculated rate higher****
**Note:**Calculated rate is lower or higher must be stated for M2.
Do not accept “scale on syringe is inaccurate”, “errors in reading syringe”, or “bubbles in syringe”.
c(i).

