- IB
- S1.1 Introduction to the particulate nature of matter
Practice S1.1 Introduction to the particulate nature of matter with authentic IB Chemistry exam questions for both SL and HL students. This question bank mirrors Paper 1A, 1B, 2 structure, covering key topics like atomic structure, chemical reactions, and organic chemistry. Get instant solutions, detailed explanations, and build exam confidence with questions in the style of IB examiners.
What change of state occurs when a gas becomes a solid without passing through the liquid phase?
Which statement correctly describes a compound?
Which statement explains why gases are compressible but solids are not?
Which of the following best distinguishes a homogeneous mixture from a heterogeneous mixture?
Which description best represents the particles in a gas?
Which statement best explains why a pure substance has a sharp melting point, whereas an impure substance does not?
A student investigates the melting behaviour of three substances: X, Y, and Z. The data collected are shown below.
Table 1. Mass of substance and temperature change during heating
| Substance | Mass / | Time to melt / | Initial temp / | Final temp / | Melting point Observed / |
|---|---|---|---|---|---|
| X | |||||
| Y | |||||
| Z |
State one piece of evidence from the data that suggests substance Y is a mixture. [1m]
Determine the average rate of temperature increase for substance Z in . [2m]
Using the data, discuss the melting behaviour of substance X and substance Y. [2m]
The substances are cooled and observed again. Only substance X reforms a solid with the same sharp melting point on reheating.
Suggest what this indicates about the type of matter in substance X. [1m]
Suggest one improvement to this experiment that would help determine whether a substance is pure more accurately. [1m]
The diagram below shows a simple apparatus used to investigate the properties of sodium chloride and carbon tetrachloride in both solid and molten states.
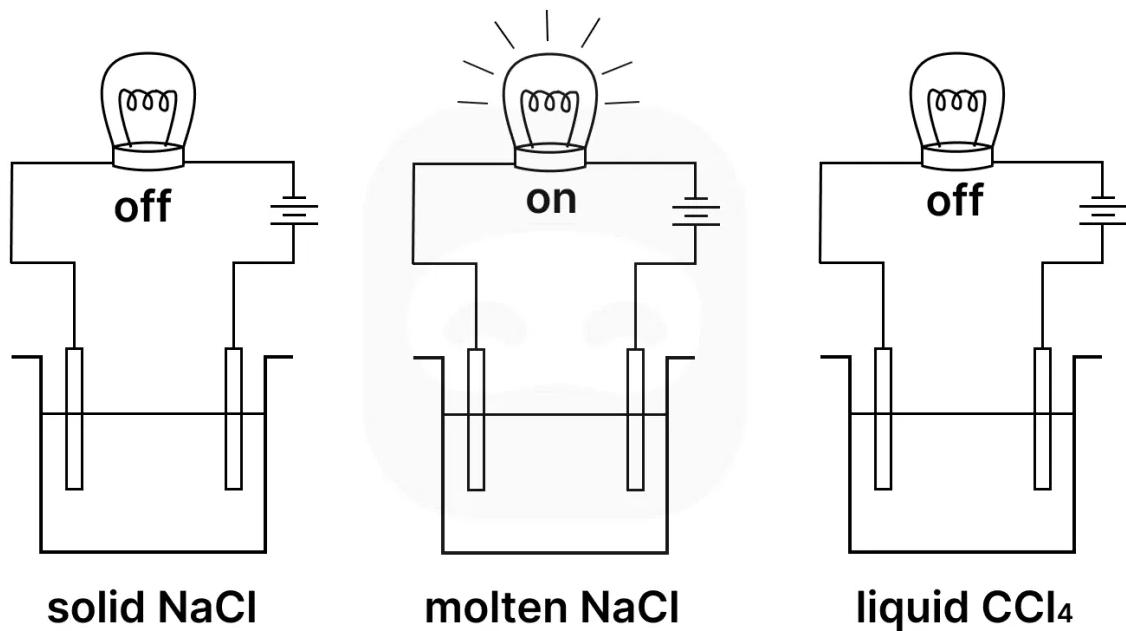
State the type of bonding present in sodium chloride and in carbon tetrachloride.
Explain why sodium chloride does not conduct electricity in the solid state but does when molten.
Suggest why carbon tetrachloride does not conduct electricity in any state.
Use the diagram and your knowledge of structure and bonding to deduce which substance(s) contain mobile ions.
State and explain the difference in melting point between sodium chloride and carbon tetrachloride.
Explain why carbon tetrachloride is a liquid at room temperature. Justify your answer based on its intermolecular forces.
Draw the Lewis (electron dot) structure for the ion and a molecule
A student is given a mixture of sodium chloride and sand. Which sequence of separation techniques would be most appropriate?
What is the sum of the coefficients when the equation for the combustion of ammonia is balanced using the smallest possible whole numbers?