Practice Structure 3. Classification of matter with authentic IB Chemistry exam questions for both SL and HL students. This question bank mirrors Paper 1A, 1B, 2 structure, covering key topics like atomic structure, chemical reactions, and organic chemistry. Get instant solutions, detailed explanations, and build exam confidence with questions in the style of IB examiners.
What trend is observed across a period in the periodic table?
Which of the following best explains the chemical similarity of the Group 17 elements?
Which compound can be oxidised when heated with an acidified solution of potassium dichromate(VI)?
The full structural formula of an organic compound is shown.
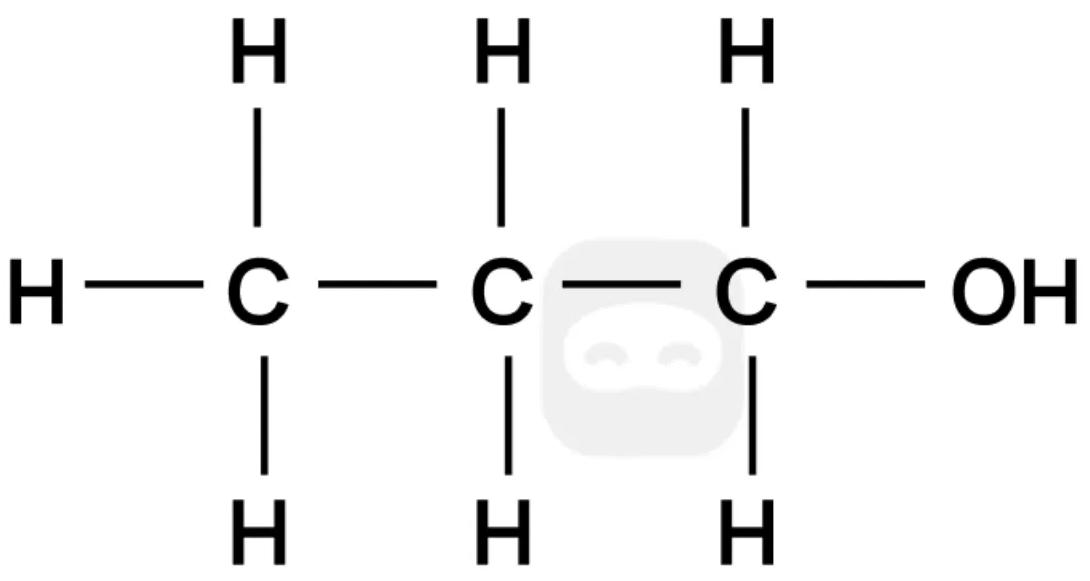
Which of the following is an isomer of the compound?
Which statements are correct for the molecule shown?
I. The molecule has a chiral centre
II. The molecule rotates the plane of plane-polarised light
III. The IUPAC name of the molecule is butan-1-ol
Which of the following structures represents an ester with molecular formula ?
Which element is in Period 4 and Group 2 of the periodic table?
Which of the following ions has the smallest radius?
The diagram shows a reaction which occurs in the production of a polymer.
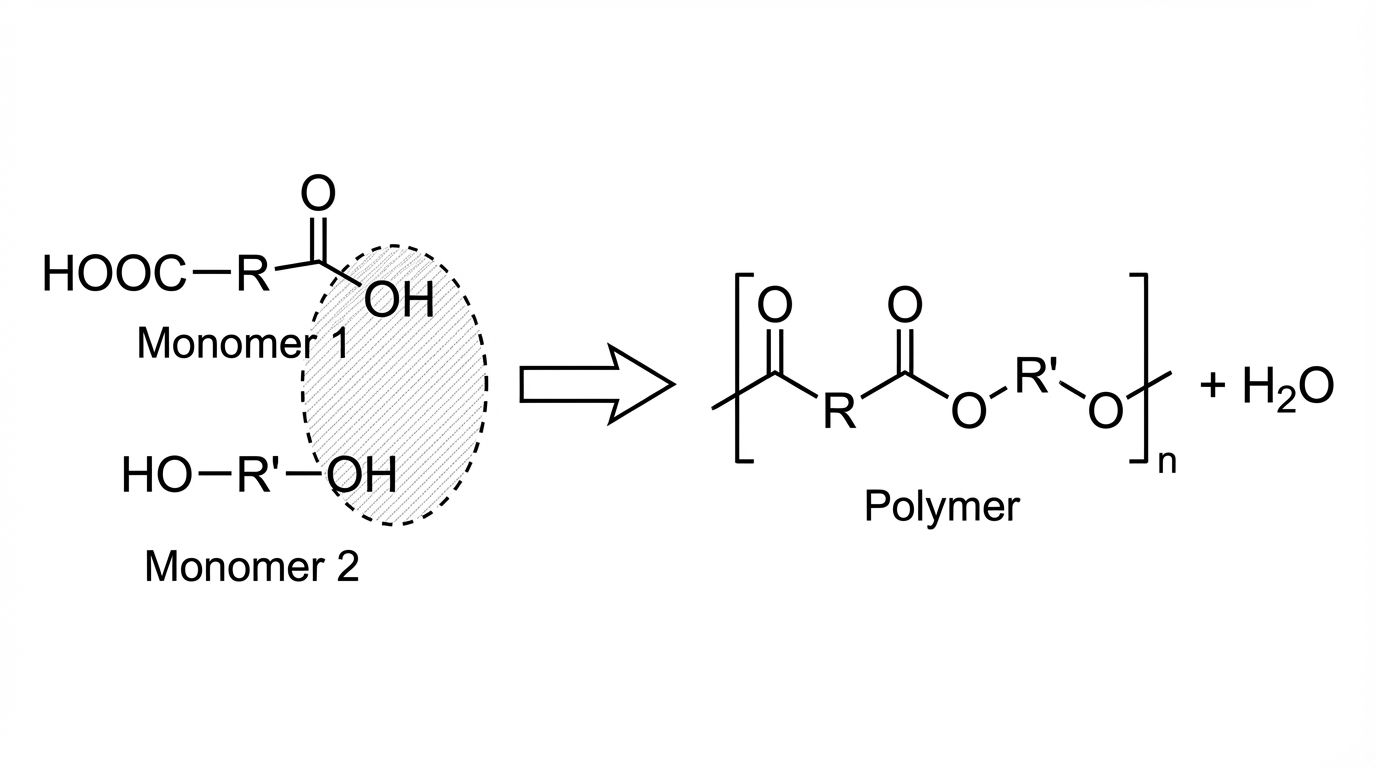
Which type of reaction is this?
A research team investigates the chemical behavior of Period 3 oxides to understand their industrial and environmental applications. The team analyzes the following oxides: , , and .
Classify the type of bonding in each of the following oxides and relate it to their position in Period 3:
Describe the trend in acid–base character of the oxides across Period 3 from sodium to chlorine.
Predict whether reacts with both acids and bases, and justify your answer using acid–base theory.