Practice R3.4 Electron-pair sharing reactions with authentic IB Chemistry exam questions for both SL and HL students. This question bank mirrors Paper 1A, 1B, 2 structure, covering key topics like atomic structure, chemical reactions, and organic chemistry. Get instant solutions, detailed explanations, and build exam confidence with questions in the style of IB examiners.
The iron(III) ion forms the complex ion shown.
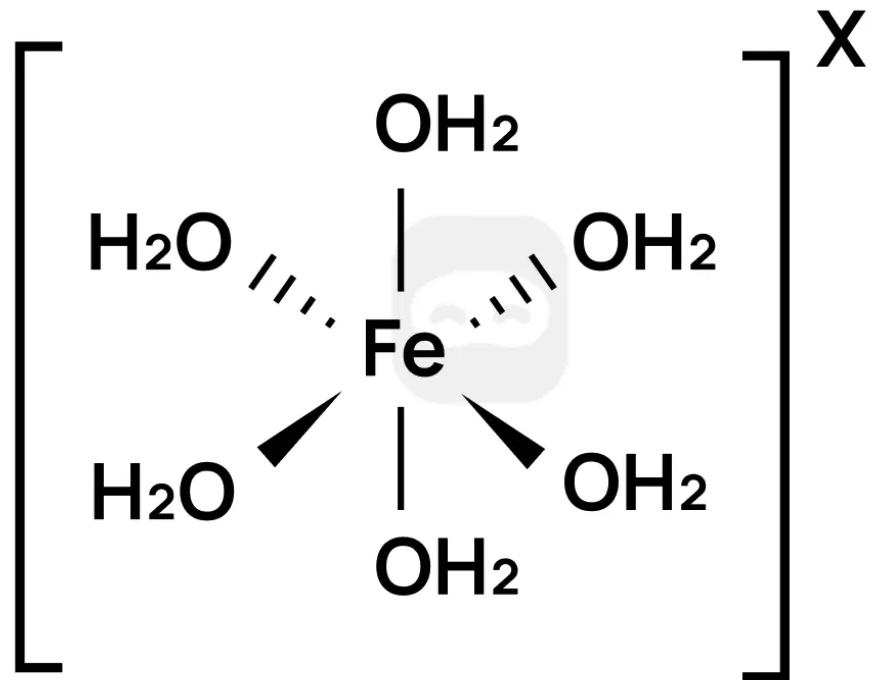
Which statements are correct?
I. The charge on the complex ion is 3+
II. The water molecules are acting as Lewis bases
III. The complex ion forms a coloured solution
Which of the following is not a nucleophile?
Which part of the haloalkane is attacked by the nucleophile?
Which of the following is the correct mechanism type for the reaction of bromoethane with aqueous sodium hydroxide?
What is the major product of the reaction between and but-2-ene?
What must be present on a nucleophile?
The diagram shows a reaction which occurs in the production of a polymer.
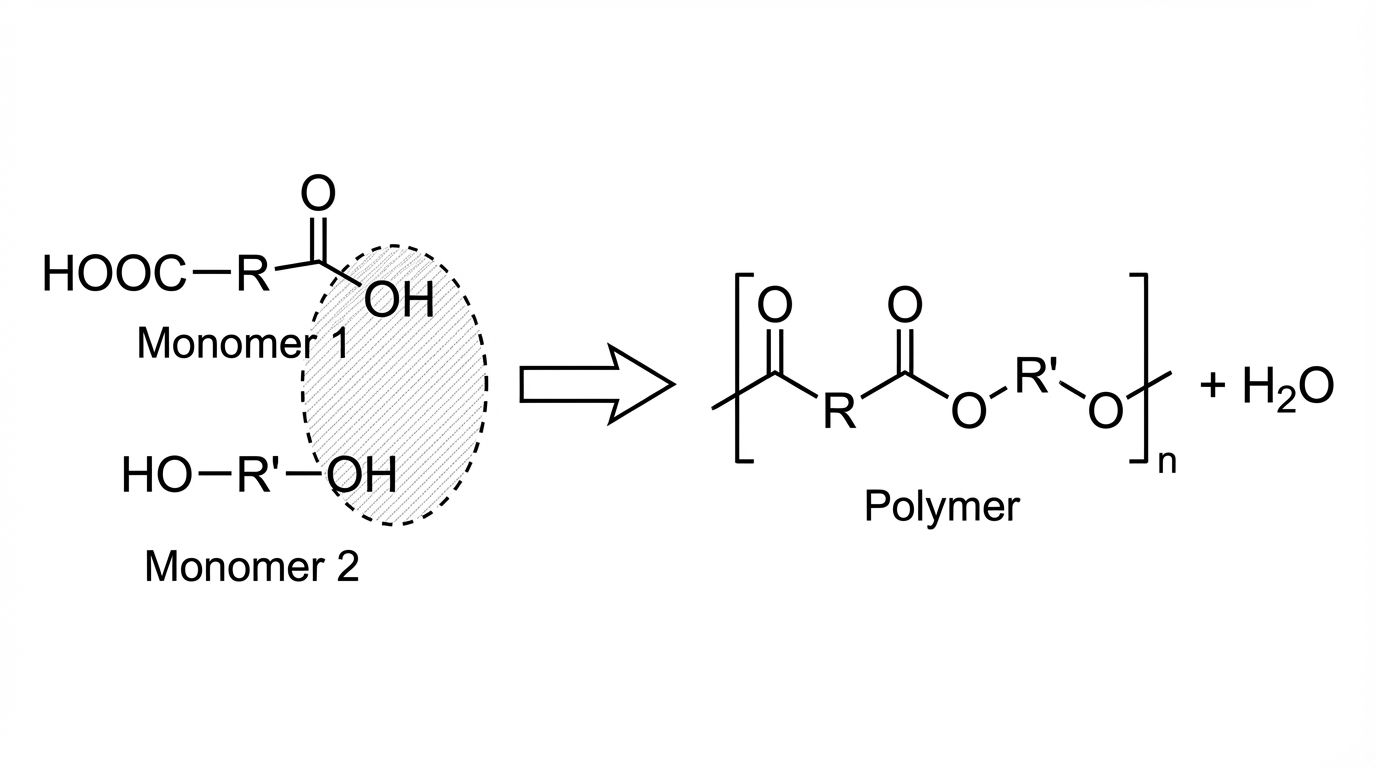
Which type of reaction is this?
The identity of the leaving group affects substitution reaction rates.
Define what is meant by a leaving group.
Compare the rate of substitution for and with the same nucleophile.
Explain your answer to part 2 with reference to bond strength.
Which is a major product of the electrophilic addition of hydrogen chloride to propene?
What type of reaction is represented by the following equation?