Practice R3.2 Electron transfer reactions with authentic IB Chemistry exam questions for both SL and HL students. This question bank mirrors Paper 1A, 1B, 2 structure, covering key topics like atomic structure, chemical reactions, and organic chemistry. Get instant solutions, detailed explanations, and build exam confidence with questions in the style of IB examiners.
The apparatus for the electrolysis of molten sodium chloride is shown.
Which statement is correct?
Given the standard electrode potentials:
Which statement is correct about the spontaneous direction of electron flow?
Which of the following is always true in a redox reaction?
Which compound can be oxidised when heated with an acidified solution of potassium dichromate(VI)?
What are the products of electrolysis when concentrated calcium bromide solution is electrolysed using graphite electrodes?
| Product at cathode (negative electrode) | Product at anode (positive electrode) | |
|---|---|---|
| A. | hydrogen | bromine |
| B. | calcium | oxygen |
| C. | calcium | bromine |
| D. | hydrogen | oxygen |
Which compound contains sulfur with an oxidation state of +6?
Voltaic cells convert chemical energy into electrical energy.
Identify the direction of electron flow in a zinc–copper voltaic cell.
State the species reduced at the cathode.
Explain why the flow of electrons occurs in that direction.
Which element undergoes reduction in the following reaction?
What is the change in the oxidation state of oxygen?
The diagram below shows a voltaic cell.
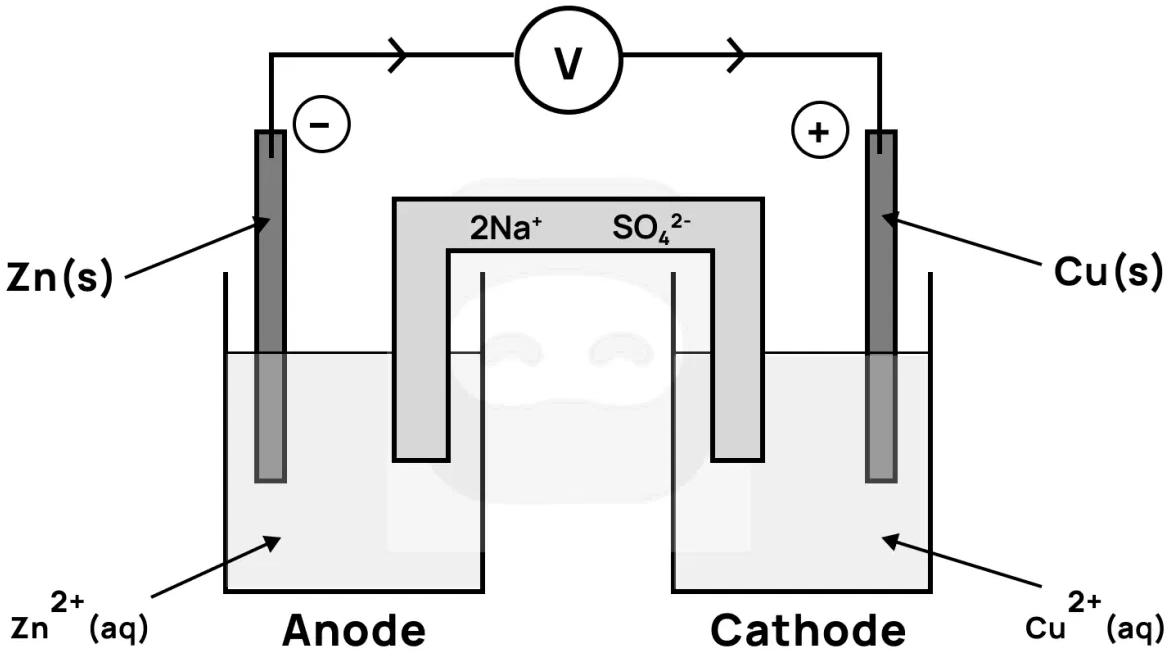
Which statement is correct?