- IB
- 1.1 Introduction to the particulate nature of matter and chemical change
Practice 1.1 Introduction to the particulate nature of matter and chemical change with authentic IB Chemistry exam questions for both SL and HL students. This question bank mirrors Paper 1A, 1B, 2 structure, covering key topics like atomic structure, chemical reactions, and organic chemistry. Get instant solutions, detailed explanations, and build exam confidence with questions in the style of IB examiners.
When this equation is balanced correctly, the coefficient, , for is
Which equation represents sublimation?
What change(s) occur(s) when a liquid boils? I. The average energy of the particles increases. II. The attractive forces between the particles become stronger. III. The spacing between the particles increases.
What is the sum of the integer coefficients when propene undergoes complete combustion?
Which graph shows how the average kinetic energy of the particles varies with absolute temperature for an ideal gas?
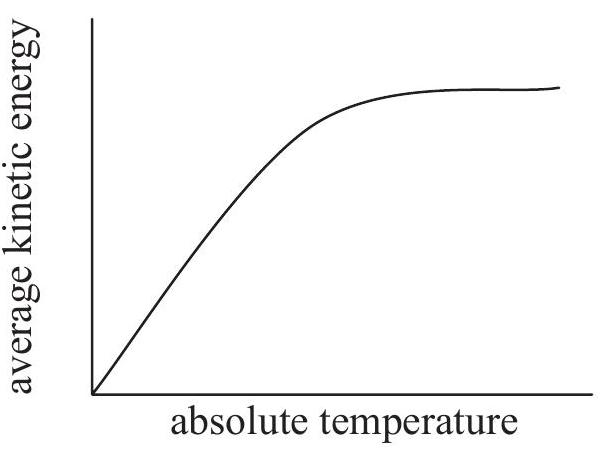
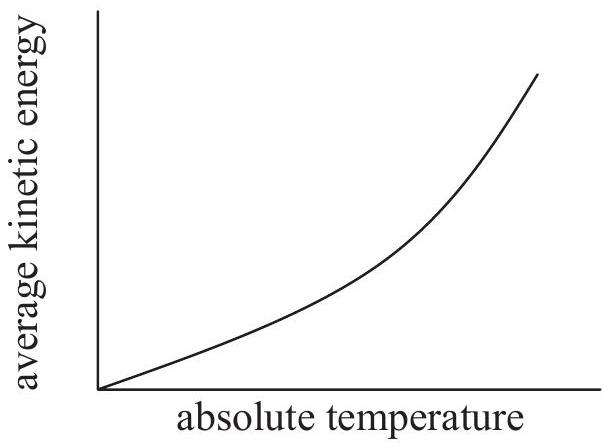
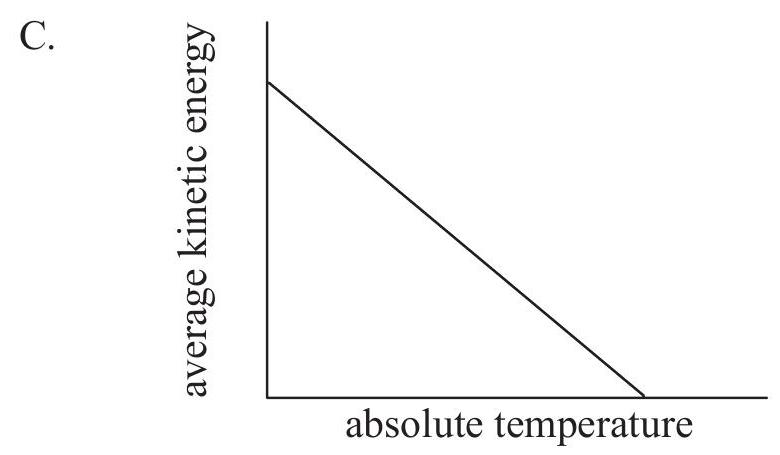
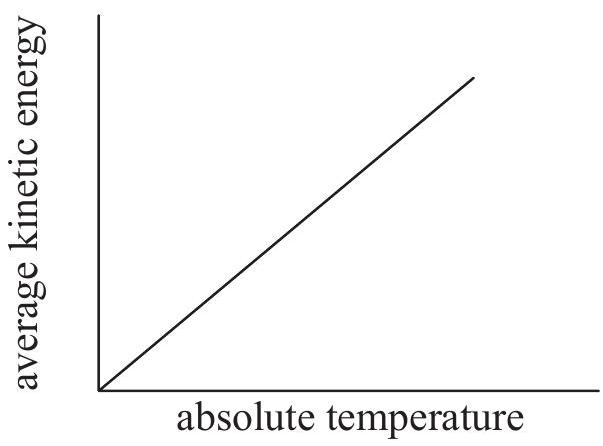
A reaction occurring in the extraction of lead from its ore can be represented by this unbalanced equation:
When the equation is balanced using the smallest possible whole numbers, what is the coefficient for ?
Which statement about the kinetic theory is not correct?
Aluminium carbide reacts with water according to the equation below. What is the sum of all the coefficients when the equation is balanced?
Which statements about mixtures are correct?
I. The components may be elements or compounds.
II. All components must be in the same phase.
III. The components retain their individual properties.
What changes occur when ice at its melting point is converted to liquid water?
I. movement of the molecules increases II. distance between molecules increases