Practice 1.3 Reacting masses and volumes with authentic IB Chemistry exam questions for both SL and HL students. This question bank mirrors Paper 1A, 1B, 2 structure, covering key topics like atomic structure, chemical reactions, and organic chemistry. Get instant solutions, detailed explanations, and build exam confidence with questions in the style of IB examiners.
What is the percentage yield when 2.0 g of ethene, , is formed from 5.0 g of ethanol, ?
(ethene) = 28; (ethanol) = 46
What is the volume of gas when the pressure on 100 of gas is changed from 800 to 200 at constant temperature?
Which volume of ethane gas, in , will produce 40 of carbon dioxide gas when mixed with 140 of oxygen gas, assuming the reaction goes to completion?
Which graph would not show a linear relationship for a fixed mass of an ideal gas with all other variables constant?
Which factors affect the molar volume of an ideal gas?
I. Pressure
II. Temperature
III. Empirical formula
What is the concentration of chloride ions, in , in a solution formed by mixing 200 of 1 HCl with 200 of 5 NaCl?
Which expression represents the density of a gas sample of relative molar mass, , at temperature, , and pressure, P ?
When this equation is balanced correctly, the coefficient, , for is
In order to determine the enthalpy change of reaction between zinc and copper(II) sulfate, a student placed 50.0 cm³ of 0.200 moldm⁻³ copper(II) sulfate solution in a polystyrene beaker. The temperature was recorded every 30 seconds. After two minutes 1.20 g of powdered zinc was added. The solution was stirred and the temperature recorded every half minute for the next 14 minutes. The results obtained were then plotted to give the following graph: 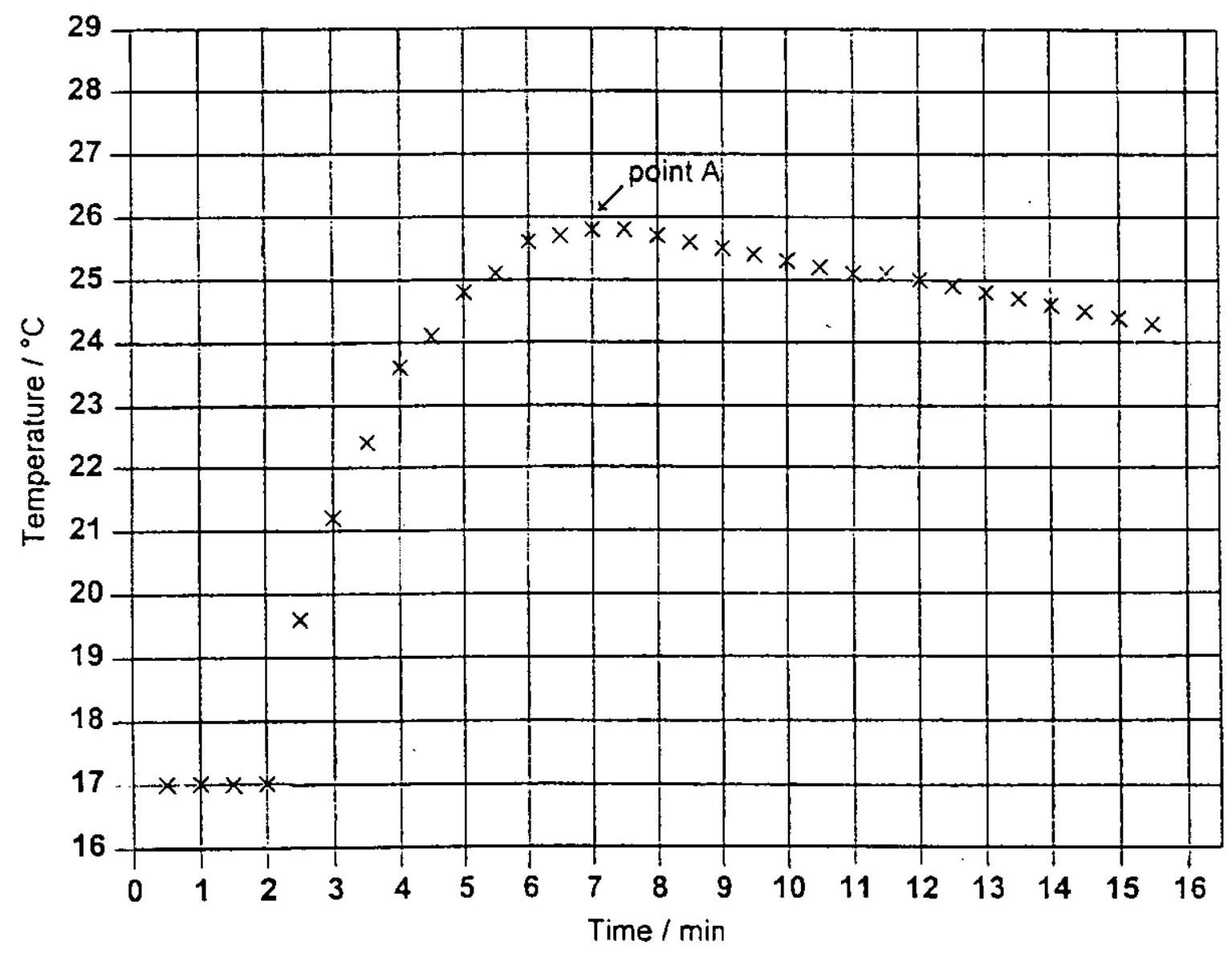
Write the equation for the reaction taking place.
Determine which of the two reagents was present in excess.
The highest temperature is reached at point A. Explain what is happening in the system at this point.
By drawing a suitable line on the graph estimate what the rise in temperature would have been if the reaction had taken place instantaneously.
Calculate how much heat was evolved during the reaction. Give your answer to three significant figures.
What is the enthalpy change of reaction in kJ mol⁻¹?
The accepted value for the enthalpy change of reaction is -218 kJ mol⁻¹. What is the percentage error for the value obtained in this experiment?
Suggest two reasons why there is disagreement between the experimental value and the accepted value.
The temperature in Kelvin of of an ideal gas is doubled and its pressure is increased by a factor of four. What is the final volume of the gas?