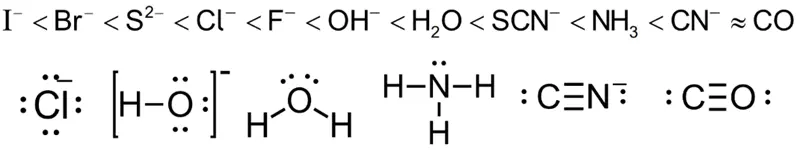Coordination Bonds and Complex Ions
What Are Coordination Bonds?
Coordination bond
A coordination bond (also known as a dative covalent bond) forms when both electrons in the shared pair come from the same atom.
In the context of complex ions, this occurs when a ligand donates a lone pair of electrons to a transition metal cation.
Ligand
A molecule or ion that donates a lone pair of electrons to a central metal ion to form a coordination bond.
Common ligands include water ($H_2O$), ammonia ($NH_3$), and chloride ions ($Cl^-$).
Other important definitions:
- Transition Metal Cation: A positively charged ion of a transition metal, which has vacant orbitals capable of accepting electron pairs from ligands.
- Complex Ion: A charged species consisting of a central metal ion surrounded by ligands bonded through coordination bonds.
Ligands must have at least one lone pair of electrons to form a coordination bond with the metal ion.
How Are Coordination Bonds Formed?
- Coordination bonds are formed when ligands donate their lone pairs of electrons to the empty orbitals of a transition metal cation.
- This process can be broken down into the following steps:
- Electron Pair Donation: The ligand, which has a lone pair of electrons, approaches the transition metal cation.
- Orbital Overlap: The lone pair of electrons from the ligand overlaps with an empty orbital on the metal ion.
- Bond Formation: A coordination bond is established, resulting in the formation of a complex ion.
Why Transition Metals?
Transition metals are particularly suited to forming complex ions because:
- They have vacant d-orbitals that can accept electron pairs.
- Their high charge density attracts electron-rich ligands.
- They can exhibit multiple oxidation states, allowing for a variety of coordination environments.
The [Cu(NH₃)₄]²⁺ Complex Ion
Let’s examine the formation of the tetraamminecopper(II) ion, $[Cu(NH_3)_4]^{2+}$, as an example of a complex ion.
- The Metal Ion:
- Copper(II) ion ($Cu^{2+}$) acts as the central metal ion.
- It has a high charge density and vacant orbitals to accept electron pairs.
- The Ligand:
- Ammonia ($NH_3$) molecules act as ligands.
- Each $NH_3$ molecule has a lone pair of electrons on the nitrogen atom.
- Bond Formation:
- Four $NH_3$ molecules donate their lone pairs to the $Cu^{2+}$ ion, forming four coordination bonds.
- This results in the complex ion $[Cu(NH_3)_4]^{2+}$.
Representation of the Complex Ion
The structure of $[Cu(NH_3)_4]^{2+}$ can be represented as:
$$
[Cu(NH_3)_4]^{2+}
$$
Here:
- The central $Cu^{2+}$ ion is surrounded by four $NH_3$ ligands.
- The overall charge of the complex ion is $+2$, which is the sum of the charge on the metal ion and the neutral ligands.
In the $[Cu(NH_3)_4]^{2+}$ complex, the copper ion accepts four lone pairs of electrons from four ammonia molecules, forming a stable coordination complex.
Determining the Charge on a Complex Ion
- To deduce the charge on a complex ion, you can use the oxidation state of the central metal ion and the charges of the ligands present.
- Ligands can be either neutral (e.g., $H_2O$, $NH_3$) or negatively charged (e.g., $Cl^-$, $CN^-$).
- The overall charge of the complex ion is the sum of the oxidation state of the metal and the charges contributed by all ligands.
In the complex ion $[\text{Fe(CN)}_6]^{4-}$:
- Cyanide ($CN^-$) carries a charge of -1 each, contributing a total charge of $-6$ ($6 \times -1$).
- The overall charge of the complex ion is -4, so the oxidation state of iron must be +2 since $+2 + (-6) = -4$.
By carefully summing the metal oxidation state and ligand charges, you can systematically determine the charge of any complex ion.
Common ions formed by the transition elements:
Properties of Complex Ions
Complex ions exhibit unique properties due to the interaction between the metal ion and the ligands. These include:
- Color: Many complex ions are colored due to electronic transitions within the d-orbitals of the metal ion.
- Magnetism: The magnetic properties of a complex ion depend on the number of unpaired electrons in the metal ion.
- Stability: The stability of a complex ion depends on factors such as the charge density of the metal ion and the nature of the ligands.
The color of a complex ion can often be used to identify the metal ion and its oxidation state.
- Do not confuse coordination bonds with ionic or covalent bonds.
- Coordination bonds involve the donation of a lone pair from a ligand to a metal ion, whereas covalent bonds involve the sharing of electrons between two atoms.
- You recap the concept of coordination bond in the article Structure 2.2.3.
- Remember that not all molecules can act as ligands.
- Only species with lone pairs of electrons can form coordination bonds.
- What is the role of a ligand in the formation of a coordination bond?
- Why are transition metals particularly suited to forming complex ions?