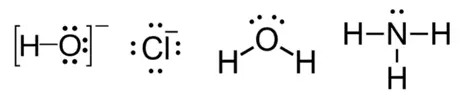Nucleophiles: Definition and Recognition
What is a Nucleophile?
Nucleophile
A nucleophile is an electron-rich species that donates a pair of electrons to form a covalent bond. The term “nucleophile” originates from the Greek words nucleus (meaning core) and philos (meaning loving), indicating its affinity for positively charged or electron-deficient centers.
Key Features of Nucleophiles:
- Electron-rich nature: Nucleophiles possess a high electron density, often due to lone pairs of electrons or a negative charge.
- Ability to donate electrons: They have lone pairs or $\pi$-electrons available for bonding.
- Attraction to electrophiles: Nucleophiles are drawn to electron-deficient species, such as positively charged ions or atoms with partial positive charges
- Negatively charged species: Hydroxide ion $OH^-$, cyanide ion $CN^-$, halide ions $Cl^-$, $Br^-$, $I^-$, and hydrosulfide ion $HS^-$.
- Neutral molecules: Ammonia $NH_3$, water $H_2O$, and methylamine $CH_3NH_2$.
- The hydroxide ion $OH^-$ is a classic nucleophile.
- It has three lone pairs of electrons on the oxygen atom, making it highly electron-rich and ready to donate an electron pair to an electrophile.
How to Recognize a Nucleophile?
- Identifying nucleophiles in a reaction requires paying attention to species with either a negative charge or lone pairs of electrons.
- These features make a species electron-rich and capable of donating electrons.
Steps to Identify Nucleophiles:
- Check for negative charges: Anions like $OH^-$, $CN^-$, and $Cl^-$ are common nucleophiles.
- Look for lone pairs: Neutral molecules such as $NH_3$ and $H_2O$ can act as nucleophiles because they have lone pairs of electrons.
- Consider the reaction context: The role of a nucleophile often depends on the reaction conditions. For instance, water $H_2O$ can act as a nucleophile in polar solvents.
- Students often confuse nucleophiles with electrophiles.
- Remember, nucleophiles are electron-rich and donate electrons, while electrophiles are electron-deficient and accept electrons.
Charged vs. Neutral Nucleophiles
Both charged and neutral species can act as nucleophiles, but their reactivity can differ significantly.
Negatively Charged Nucleophiles:
- Examples: $OH^-$, $CN^-$, $Cl^-$, $Br^-$, $I^-$.
- These are generally stronger nucleophiles because their negative charge increases their electron density, making them more reactive.
Neutral Nucleophiles:
- Examples: $NH_3$, $H_2O$, $CH_3NH_2$.
- These are weaker nucleophiles compared to their charged counterparts but can still participate in reactions due to their lone pairs of electrons.
- The strength of a nucleophile depends on its ability to donate electrons.
- Factors such as charge, electronegativity, and the solvent can influence nucleophilicity.
- For instance, $OH^-$ is a stronger nucleophile than $H_2O$ because of its negative charge.