Dynamic Equilibrium: A Balance in Motion
Dynamic equilibrium
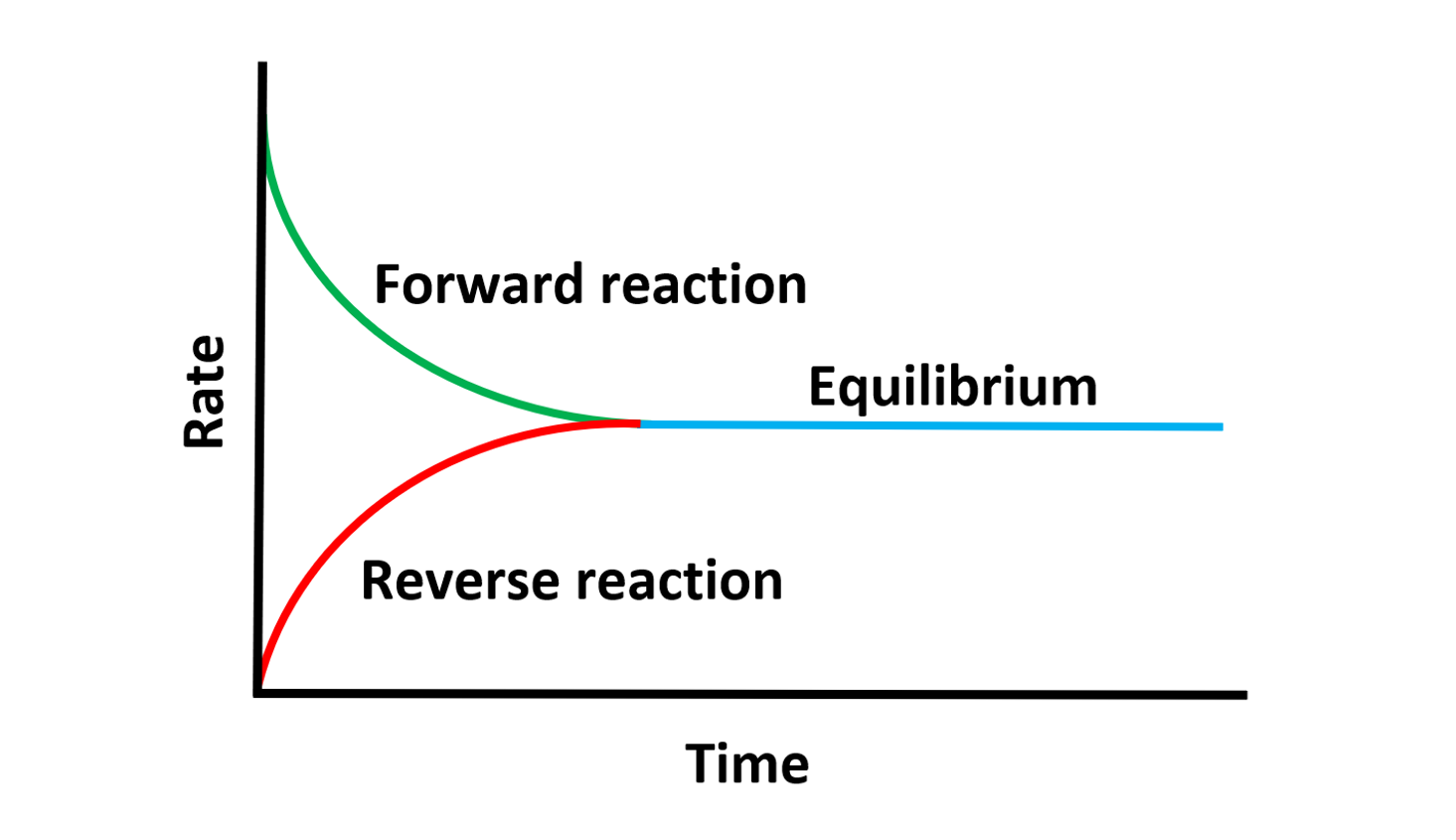
Dynamic equilibrium occurs in reversible reactions within a closed system.
Reversible reaction
A reversible reaction is one in which reactants can form products (the forward reaction), while products can simultaneously revert back into reactants (the reverse reaction).
At equilibrium, the rates of the forward and reverse reactions are equal, creating a balance.
Key Characteristics of Dynamic Equilibrium:
- Constant Concentrations:
- While reactions continue to occur, the concentrations of reactants and products remain constant over time.
- However, this does not mean the concentrations are equal—only that they are unchanging.
- Dynamic Nature:
- At the molecular level, particles are continuously reacting.
- The system is not static; it is in constant motion.
- Closed System:
- The system must be closed, meaning no reactants or products can enter or leave.
- This ensures equilibrium is maintained.
- Dynamic equilibrium is a balance of reaction rates, not amounts.
- The concentrations of reactants and products may differ, but their rates of change are always equal at equilibrium.
Examples of Dynamic Equilibrium
Phase Equilibrium: Water in a Sealed Container
- Picture a sealed container with liquid water and its vapor.
- Initially, water molecules evaporate into the gas phase, increasing the concentration of water vapor.
- Over time, some vapor molecules condense back into liquid water.
- Eventually, the rate of evaporation equals the rate of condensation, and the system reaches equilibrium.
- Equation:$$
\text{H}_2\text{O}(l) \rightleftharpoons \text{H}_2\text{O}(g)
$$
At equilibrium:
- The liquid level appears constant.
- The concentration of water vapor stabilizes.
Chemical Equilibrium: The Haber Process
- A classic example of chemical equilibrium is the synthesis of ammonia in the Haber process:
- Equation:$$
\text{N}_2(g) + 3\text{H}_2(g) \rightleftharpoons 2\text{NH}_3(g)
$$ - Initially, nitrogen ($ \text{N}_2 $) and hydrogen ($ \text{H}_2 $) combine to form ammonia ($ \text{NH}_3 $).
- As ammonia accumulates, the reverse reaction begins, where ammonia breaks down into nitrogen and hydrogen.
- Eventually, the rates of synthesis and decomposition equalize, and the concentrations of all three gases remain constant.
At equilibrium:
- Reactants ($ \text{N}_2 $ and $ \text{H}_2 $) and products ($ \text{NH}_3 $) coexist.
- The reaction continues in both directions at equal rates.
- Students often assume that equilibrium means the concentrations of reactants and products are equal.
- This is incorrect, because only the rates of the forward and reverse reactions are equal.
Achieving Equilibrium: A Closer Look
Starting with Reactants
- If a reaction begins with only reactants, the forward reaction dominates initially, producing products.
- As products accumulate, the reverse reaction begins.
- Over time, the rates of the forward and reverse reactions equalize, and equilibrium is established.
Starting with Products
- Equilibrium can also be reached starting with only products.
- In this case, the reverse reaction dominates initially, converting products into reactants.
- Eventually, the same equilibrium state is reached, regardless of the starting conditions.
- Consider the reaction: $$
\text{2NH}_3(g) \rightleftharpoons \text{N}_2(g) + 3\text{H}_2(g)
$$ - If you start with 1.00 mol of $ \text{NH}_3 $, the system will reach the same equilibrium concentrations as if you had started with 0.50 mol of $ \text{N}_2 $ and 1.50 mol of $ \text{H}_2 $.
- Why is a closed system necessary for dynamic equilibrium?
- How would an open system affect the ability to reach equilibrium?