- IB
- R2.3 How far? The extent of chemical change
Practice R2.3 How far? The extent of chemical change with authentic IB Chemistry exam questions for both SL and HL students. This question bank mirrors Paper 1A, 1B, 2 structure, covering key topics like atomic structure, chemical reactions, and organic chemistry. Get instant solutions, detailed explanations, and build exam confidence with questions in the style of IB examiners.
Which of the following would result in a decrease in the equilibrium constant for a reversible reaction?
Which statement is correct about a system at equilibrium?
For the reaction
Which change will decrease the value of ?
What is the percentage yield when 7 g of ethene produces 6 g of ethanol?
and
Which factor does not affect the position of equilibrium in this reaction?
The following infrared (IR) spectrum is for 2-butanone, an organic compound with the molecular formula .
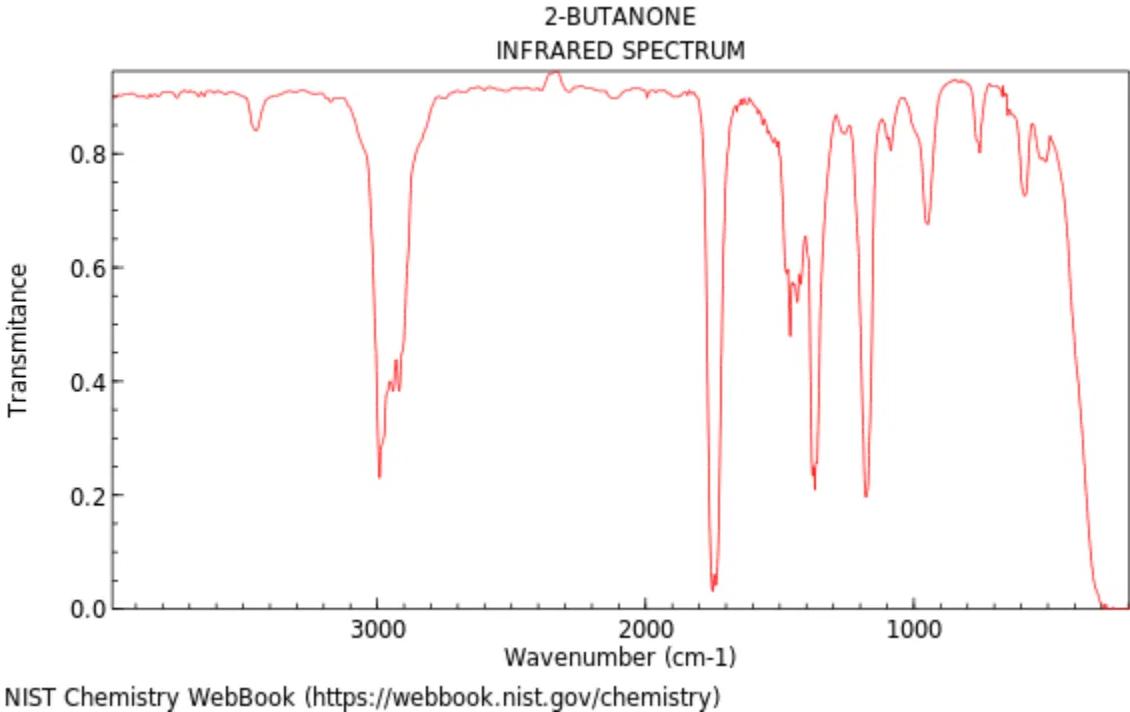
Identify the functional group present in 2-butanone that corresponds to the strong absorption peak around .
Predict why there is no broad absorption peak in the region around .
Discuss the significance of the multiple sharp peaks appearing between in organic structure identification.
The IR spectrum of 2-butanone was recorded to monitor a chemical reaction.
Explain how this spectrum could be used to determine whether 2-butanol has been fully oxidized to 2-butanone.
Describe how the IR spectra of 2-butanone and butanoic acid would differ, and explain how these differences arise.
For the reaction:
Which combination of changes will shift the equilibrium position most to the right?
A sealed container holds a sample of water in equilibrium with water vapour. What happens to the number of water vapour molecules if the temperature increases?
The reaction:
was studied with the following initial concentrations:
, , , and .
Write the expression for the reaction quotient .
Calculate the value of .
Given that , predict the direction the reaction will proceed to reach equilibrium.
Hydrogen peroxide decomposes in aqueous solution according to the reaction:
The concentration of hydrogen peroxide was measured over time. The graph below shows the concentration of decreasing during the reaction.
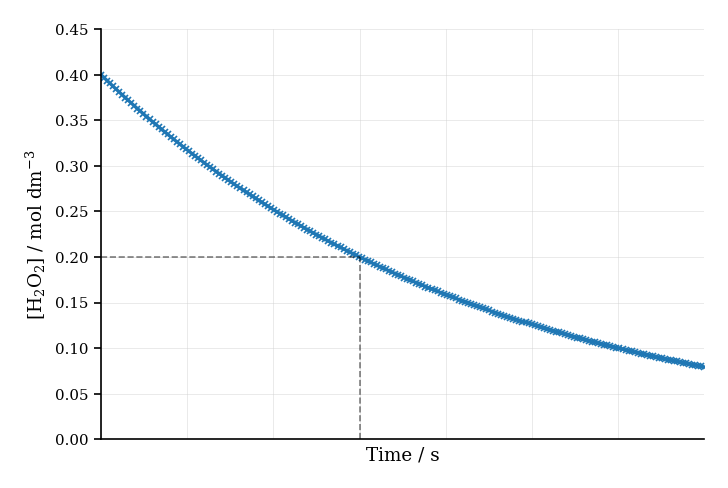
Explain how the rate of reaction can be determined from the graph at a specific time.
Suggest why the curve flattens out as the reaction proceeds.
Calculate the average rate of reaction between and seconds, given that falls from to .
Describe how you would determine the initial rate of reaction using the graph.
The rate equation for the decomposition is:
Explain how you could determine the order of reaction, , from experimental data.
Sketch a graph of versus time for a first-order reaction.