Polar and Non-Polar Amino Acids Shape Protein Folding and Stability
- Amino acids in proteins can be classified into two groups based on their properties:
- Polar (hydrophilic) or,
- Non-polar (hydrophobic).
- This distinction plays a fundamental role in determining how proteins fold into their functional three-dimensional shapes.
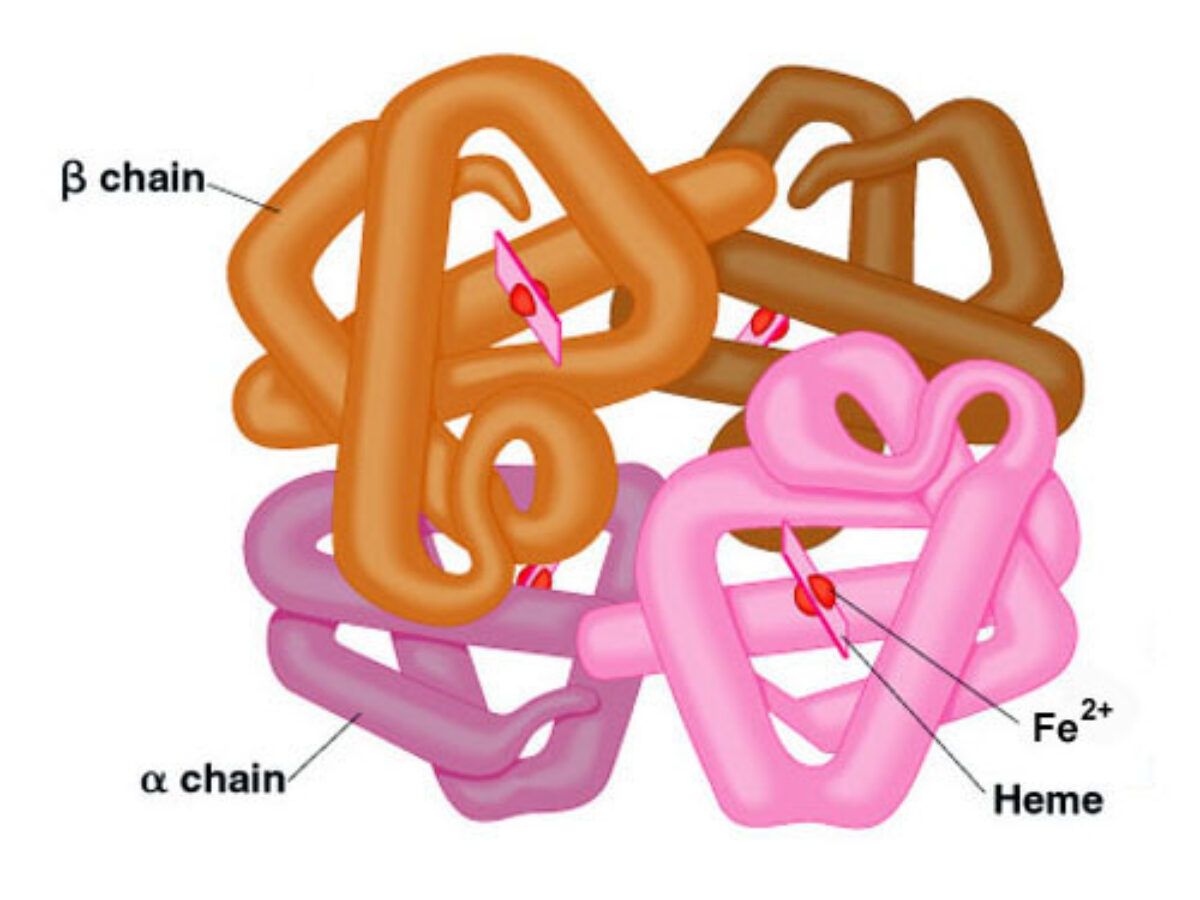
- Hemoglobin, the protein responsible for oxygen transport in red blood cells, demonstrates the importance of these properties.
- Its hydrophobic amino acids are sequestered inside the protein, stabilizing its structure, while hydrophilic amino acids are exposed on the surface, allowing interaction with the watery environment of the blood.
Polar Amino Acids
- Polar amino acids are hydrophilic, meaning they interact readily with water.
- They are typically found on the surface of globular proteins, where they can form hydrogen bonds with water or other molecules.
- This arrangement enhances the solubility of the protein and enables interactions with the aqueous environment.
In transmembrane proteins, polar amino acids line the channel, facilitating the passage of polar or charged molecules through the membrane.
Non-Polar Amino Acids
- Non-polar amino acids are hydrophobic, meaning they avoid water.
- They tend to cluster together in the interior of globular proteins, forming a stable hydrophobic core.
- This configuration minimizes exposure to water and drives the folding of the protein into its tertiary structure.
- In membrane proteins, non-polar amino acids interact with the hydrophobic fatty acid tails of the lipid bilayer, anchoring the protein within the membrane.
How They Shape Protein Structure
- In water-soluble proteins:
- Hydrophobic amino acids stabilize the protein’s interior by clustering together.
- Hydrophilic amino acids maintain interactions with water on the surface.
- In membrane proteins:
- Hydrophobic regions anchor the protein in the lipid bilayer.
- Hydrophilic regions interact with aqueous environments inside and outside the cell.
Channel Proteins
- Channel proteins illustrate the interplay of polar and non-polar amino acids.
- Hydrophobic regions anchor the protein in the membrane.
- Hydrophilic regions line the channel, enabling water and polar molecules to pass through.
- How does the clustering of hydrophobic amino acids relate to entropy in thermodynamics?
- Reflect on how the arrangement of water molecules changes when hydrophobic amino acids group together.
- Why are hydrophobic amino acids typically found in the core of globular proteins?
- How do membrane proteins differ in their distribution of polar and non-polar amino acids compared to water-soluble proteins?
- What role do hydrogen bonds play in stabilizing protein structure?