Components of the Atom and Nuclear Symbols
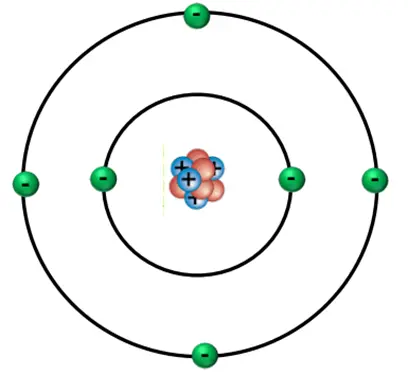
The Components of the Atom
- Atoms are the fundamental building blocks of matter, but they are not indivisible.
- They consist of three types of subatomic particles: protons, neutrons, and electrons.
- Each of these particles has distinct properties and plays a specific role in the atom.
The Nucleus: Protons and Neutrons
At the heart of the atom lies the nucleus.
Nucleus
A nucleus is a dense, positively charged core that contains protons and neutrons, collectively called nucleons.
- Protons:
- These are positively charged particles with a relative charge of $ +1 $ and a relative mass of $ 1 $.
- The number of protons in the nucleus determines the atomic number (Z), which defines the element.
- Neutrons:
- These particles are neutral, with no charge, and have a relative mass of $ 1 $.
- Neutrons help to stabilize the nucleus by offsetting the repulsion between protons.
- The number of neutrons can vary between atoms of the same element, leading to isotopes.
Protons and neutrons are approximately 1,836 times more massive than electrons, meaning nearly all the mass of an atom is concentrated in its nucleus.
2.The Energy Levels
- Surrounding the nucleus is a vast region of space occupied by electrons, which are negatively charged particles with a relative charge of $ -1 $.
- Electrons have a negligible mass compared to protons and neutrons.
- Electrons are arranged in regions of space called orbitals, which define the probability of finding an electron in a certain area.
- In a neutral atom, the number of electrons equals the number of protons, balancing the positive and negative charges.
Structure of Atom
Relative Masses and Charges of Subatomic Particles
- To understand the behavior of atoms, it’s important to know the relative masses and charges of their subatomic particles.
- These are summarized in the table below:
| Particle | Relative Mass | Relative Charge | Location |
|---|---|---|---|
| Proton | 1 | +1 | Nucleus |
| Neutron | 1 | 0 | Nucleus |
| Electron | Negligible | -1 | Outside the nucleus |
The mass of an electron is so small compared to protons and neutrons that it is often considered negligible in calculations.
Nuclear Symbols: Decoding the Atom’s Identity
- Chemists use nuclear symbols to summarize the structure of an atom or ion.
- A nuclear symbol is written in the form: $$ ^A_Z X $$ where:
- $ X $ = Chemical symbol of the element (e.g., $ \text{Au} $ for gold).
- $ Z $ = Atomic number (number of protons).
- $ A $ = Mass number (total number of protons and neutrons).
Gold
For gold, the nuclear symbol is:
$$
^{197}_{79} \text{Au}
$$
This tells us:
- $ Z = 79 $: Gold has 79 protons.
- $ A = 197 $: Gold has a total of 197 nucleons.
- Number of neutrons = $ A - Z = 197 - 79 = 118 $.
Can you determine the number of protons, neutrons, and electrons in $ ^{23}_{11} \text{Na} $?
Atomic Symbols & Nuclear Notation
Atoms vs. Ions: What Happens When Electrons Change?
While atoms are neutral, they can gain or lose electrons to form ions.
Ion
An ion is an atom or group of atoms that has gained or lost electrons, resulting in a net electrical charge.
This does not affect the number of protons or neutrons but changes the overall charge of the species.
- Cations: Formed when an atom loses electrons, resulting in a positive charge.
- Anions: Formed when an atom gains electrons, resulting in a negative charge.
Magnesium ion ($ \text{Mg}^{2+} $) has 12 protons but only 10 electrons.
An oxide ion ($ \text{O}^{2-} $) has 8 protons and 10 electrons.
Deduce the nuclear symbol for an ion with:
- 24 protons
- 21 electrons
- 28 neutrons
Solution
- The atomic number ($ Z $) is 24, so the element is chromium ($ \text{Cr} $).
- The mass number ($ A $) is $ 24 + 28 = 52 $.
- The ion has $ 24 - 21 = +3 $ charge.
- The nuclear symbol is:$$^{52}_{24} \text{Cr}^{3+}$$
- Magnesium forms a $ \text{Mg}^{2+} $ ion by losing two electrons.
- Its nuclear symbol is $ ^{24}_{12} \text{Mg}^{2+} $.
- This indicates 12 protons, 12 neutrons ($ 24 - 12 = 12 $), and 10 electrons.
Atomic Structure Game
- Students often confuse the mass number ($ A $) with the atomic number ($ Z $).
- Remember: $ A $ includes both protons and neutrons, while $ Z $ is just the number of protons.