Reversibility of Redox Reactions in Electrochemical Cells
- In chemistry, a redox reaction involves the transfer of electrons between two species.
- One species is oxidized (loses electrons), while the other is reduced (gains electrons).
- In some cases, these reactions are reversible, meaning the products can be converted back into reactants by applying an external energy source, such as an electric current.
How Reversibility Works
- Reversibility in redox reactions is the basis of secondary (rechargeable) electrochemical cells.
- During discharge, the cell converts chemical energy into electrical energy through spontaneous redox reactions.
- When an external voltage is applied during charging, the redox reactions are reversed, restoring the reactants.
- Discharge: Spontaneous redox reactions produce electrical energy.
- Charge: Non-spontaneous redox reactions occur, driven by external electrical energy.
To reverse a redox reaction in a rechargeable battery, the applied voltage must be slightly greater than the cell’s standard voltage to overcome energy losses due to resistance and inefficiencies.
What is the difference between a spontaneous and a non-spontaneous redox reaction?
Examples of Reversible Redox Systems
Lead-Acid Batteries
- Lead-acid batteries are commonly used in cars and backup power systems.
- These batteries consist of a lead anode (negative electrode) and a lead(IV) oxide cathode (positive electrode) immersed in sulfuric acid.
Discharge (Powering the Device)
During discharge, the following reactions occur:
- Anode (Oxidation): $$ \text{Pb(s)} + \text{HSO}_4^-(aq) \rightarrow \text{PbSO}_4(s) + \text{H}^+(aq) + 2e^- $$
- Cathode (Reduction): $$ \text{PbO}_2(s) + 3\text{H}^+(aq) + \text{HSO}_4^-(aq) + 2e^- \rightarrow \text{PbSO}_4(s) + 2\text{H}_2\text{O}(l) $$
- Overall Cell Reaction: $$ \text{Pb(s)} + \text{PbO}_2(s) + 2\text{H}_2\text{SO}_4(aq) \rightarrow 2\text{PbSO}_4(s) + 2\text{H}_2\text{O}(l) $$
Charge (Recharging the Battery)
When an external voltage is applied, the reactions are reversed:
- Anode (Reduction): $$ \text{PbSO}_4(s) + \text{H}^+(aq) + 2e^- \rightarrow \text{Pb(s)} + \text{HSO}_4^-(aq) $$
- Cathode (Oxidation): $$ \text{PbSO}_4(s) + 2\text{H}_2\text{O}(l) \rightarrow \text{PbO}_2(s) + 3\text{H}^+(aq) + \text{HSO}_4^-(aq) + 2e^- $$
- Overall Cell Reaction: $$ 2\text{PbSO}_4(s) + 2\text{H}_2\text{O}(l) \rightarrow \text{Pb(s)} + \text{PbO}_2(s) + 2\text{H}_2\text{SO}_4(aq) $$
For instance, when you start your car, the lead-acid battery discharges to power the starter motor. As the engine runs, the alternator recharges the battery by reversing the redox reactions.
Nickel-Cadmium (NiCd) Cells
- Nickel-cadmium cells are another type of rechargeable battery, often used in portable electronic devices and power tools.
- These batteries consist of a cadmium anode and a nickel(III) oxide-hydroxide cathode in an alkaline electrolyte (usually potassium hydroxide).
Discharge (Powering the Device)
During discharge, the reactions are:
- Anode (Oxidation): $$ \text{Cd(s)} + 2\text{OH}^-(aq) \rightarrow \text{Cd(OH)}_2(s) + 2e^- $$
- Cathode (Reduction): $$ \text{NiO(OH)}(s) + \text{H}_2\text{O}(l) + e^- \rightarrow \text{Ni(OH)}_2(s) + \text{OH}^-(aq) $$
- Overall Cell Reaction: $$ \text{Cd(s)} + 2\text{NiO(OH)}(s) + 2\text{H}_2\text{O}(l) \rightarrow \text{Cd(OH)}_2(s) + 2\text{Ni(OH)}_2(s) $$
Charge (Recharging the Battery)
When an external voltage is applied, the reactions are reversed:
- Anode (Reduction): $$ \text{Cd(OH)}_2(s) + 2e^- \rightarrow \text{Cd(s)} + 2\text{OH}^-(aq) $$
- Cathode (Oxidation): $$ \text{Ni(OH)}_2(s) + \text{OH}^-(aq) \rightarrow \text{NiO(OH)}(s) + \text{H}_2\text{O}(l) + e^- $$
- Overall Cell Reaction: $$ \text{Cd(OH)}_2(s) + 2\text{Ni(OH)}_2(s) \rightarrow \text{Cd(s)} + 2\text{NiO(OH)}(s) + 2\text{H}_2\text{O}(l) $$
- NiCd batteries are durable and can endure many charge-discharge cycles, but they suffer from the "memory effect," where incomplete discharges reduce their capacity over time.
- To avoid this, fully discharge the battery periodically.
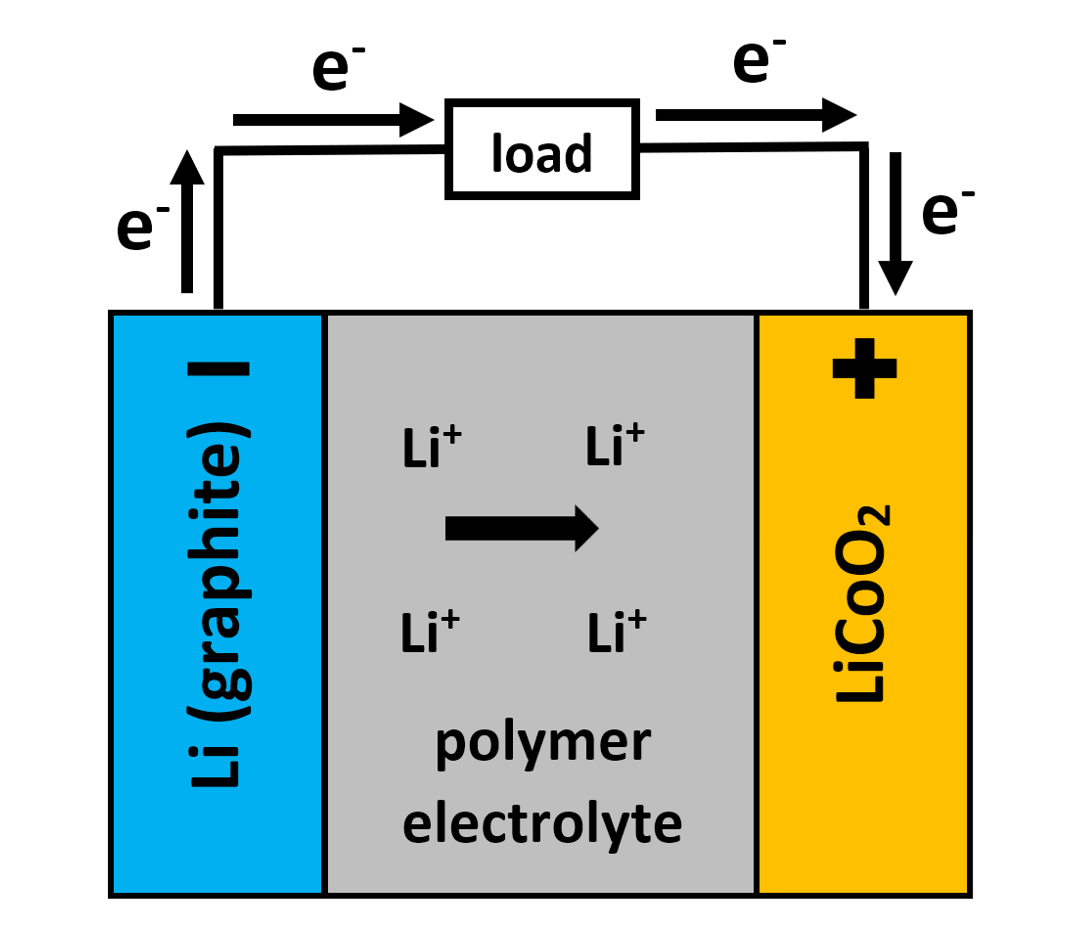
Lithium-Ion Batteries
- Lithium-ion batteries are commonly used in portable electronics, electric vehicles, and energy storage systems due to their high energy density and rechargeability.
- These batteries consist of a lithium cobalt oxide (LiCoO₂) cathode and a graphite anode, with a lithium salt electrolyte.
Discharge (Powering the Device)
During discharge, lithium ions move from the anode to the cathode, generating electrical energy:
- Anode (Oxidation):
$$\text{LiC}_6 \rightarrow C_6 + \text{Li}^+ + e^-$$ - Cathode (Reduction):
$$\text{LiCoO}_2 + \text{Li}^+ + e^- \rightarrow \text{Li}_2\text{CoO}_2$$ - Overall Cell Reaction:
$$\text{LiC}_6 + \text{LiCoO}_2 \rightarrow C_6 + \text{Li}_2\text{CoO}_2$$
Charge (Recharging the Battery)
During charging, an external power source drives the reverse reactions, storing energy by moving lithium ions back to the anode:
- Anode (Reduction):
$$C_6 + \text{Li}^+ + e^- \rightarrow \text{LiC}_6$$ - Cathode (Oxidation):
$$\text{Li}_2\text{CoO}_2 \rightarrow \text{LiCoO}_2 + \text{Li}^+ + e^-$$ - Overall Cell Reaction:
$$C_6 + \text{Li}_2\text{CoO}_2 \rightarrow \text{LiC}_6 + \text{LiCoO}_2$$
Advantages and Disadvantages of Fuel Cells, Primary Cells, and Secondary Cells
| Fuel cells | Primary cells | Secondary cells | |
|---|---|---|---|
| Advantages | High efficiency, low emissions (water as the only byproduct in hydrogen fuel cells), and continuous operation as long as fuel is supplied. | Convenient, portable, and long shelf life, making them ideal for single-use devices like remote controls and flashlights. | Reusable through multiple charge cycles, reducing waste and long-term costs, commonly used in laptops, phones, and electric vehicles. |
| Disadvantages | High production costs, storage challenges for hydrogen gas, and reliance on rare catalysts like platinum. | Non-rechargeable, contributing to electronic waste, and limited energy capacity. | Higher initial cost, capacity degradation over time, and some materials (e.g., lithium) can be environmentally harmful. |
- How do rechargeable batteries contribute to reducing fossil fuel dependency in transportation and energy storage?
- Why do rechargeable batteries eventually fail, even though their reactions are theoretically reversible? Can you propose solutions to extend their lifespan?