Electroplating and Electrolytic Cells
Electroplating
Electroplating is a process that uses an electrolytic cell to coat an object (usually the cathode) with a thin layer of metal.
By passing an electric current through an electrolyte solution containing metal ions, we can deposit a layer of metal onto the object.
To coat a steel ring with copper:
- The electrolyte would be a solution of copper(II) sulfate $ \text{CuSO}_4 $.
- The cathode (negative electrode) would be the steel ring.
- The anode (positive electrode) would be a piece of copper metal.
This setup allows copper ions from the solution to deposit onto the steel ring, while copper atoms from the anode replenish the ions in the solution.
The Role of Electrodes in Electroplating
In an electrolytic cell, the roles of the electrodes can be summarized as follows:
- Anode (Positive Electrode):
- Oxidation occurs here, where metal atoms lose electrons and form ions that enter the solution.
- Cathode (Negative Electrode):
- Reduction occurs here, where metal ions from the solution gain electrons and deposit as solid metal onto the object.
Writing Half-Equations for Electroplating
- To understand the chemical changes, we write half-equations for the reactions at each electrode.
- Let’s use the example of copper electroplating:
At the Cathode (Reduction):
The steel ring acts as the cathode, where copper ions $ \text{Cu}^{2+} $ in the solution are reduced to solid copper $ \text{Cu}(s) $:
$$
\text{Cu}^{2+} (\text{aq}) + 2e^- \to \text{Cu}(s)
$$
This equation illustrates how copper ions gain two electrons to form solid copper, which adheres to the steel ring.
At the Anode (Oxidation):
The copper anode is oxidized, releasing copper ions into the solution:
$$
\text{Cu}(s) \to \text{Cu}^{2+} (\text{aq}) + 2e^-
$$
This equation shows that solid copper loses two electrons, forming copper ions that replenish the electrolyte.
The Overall Reaction
When the two half-equations are combined, the electrons cancel out, resulting in the overall reaction:
$$
\text{Cu}(s) \text{(anode)} \to \text{Cu}(s) \text{(cathode)}
$$
This demonstrates that copper is transferred from the anode to the cathode, with the electrolyte acting as a medium for ion transfer.
In an ideal electroplating process, the rate of oxidation at the anode equals the rate of reduction at the cathode, keeping the concentration of metal ions in the electrolyte constant.
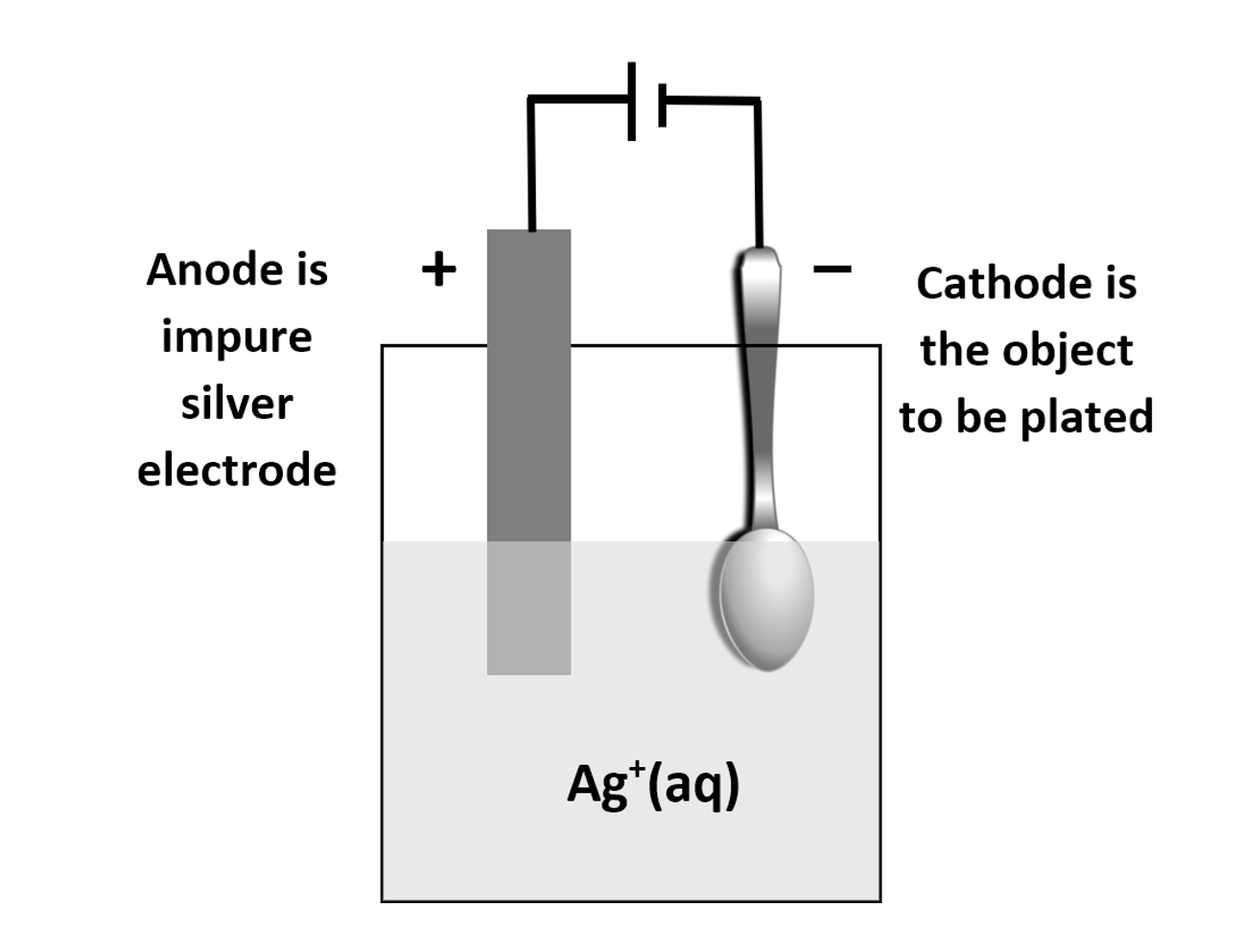
Electroplating a Nickel Spoon with Silver
Let’s explore another example: electroplating a nickel spoon with silver using a silver nitrate $ \text{AgNO}_3 $ solution.
At the Cathode (Nickel Spoon):
Silver ions $ \text{Ag}^+ $ in the solution are reduced to solid silver $ \text{Ag}(s) $:
$$
\text{Ag}^+ (\text{aq}) + e^- \to \text{Ag}(s)
$$
At the Anode (Silver Electrode):
Silver metal is oxidized to silver ions $ \text{Ag}^+ $:
$$
\text{Ag}(s) \to \text{Ag}^+ (\text{aq}) + e^-
$$
Overall Reaction:
$$
\text{Ag}(s) \text{(anode)} \to \text{Ag}(s) \text{(cathode)}
$$
In this process, silver is transferred from the anode to the nickel spoon, creating a thin, decorative, and protective layer of silver.
- What is the purpose of the electrolyte in the electroplating process?
- A gold ring is electroplated with platinum using a platinum anode and an electrolyte containing $ \text{PtCl}_6^{2-} $ ions. Write the half-equations for the reactions at the cathode and anode.