Reduction of Carboxylic Acids to Primary Alcohols and Ketones to Secondary Alcohols
Reduction in organic chemistry
Reduction in organic chemistry refers to the gain of hydrogen atoms or the loss of oxygen atoms in a molecule.
- This is the opposite of oxidation, which involves the loss of hydrogen or the gain of oxygen.
- In the context of functional groups, reduction often involves converting a more oxidized group (like a carboxylic acid or ketone) into a less oxidized one (like an alcohol).
Reduction of Carboxylic Acids to Primary Alcohols (via Aldehydes)
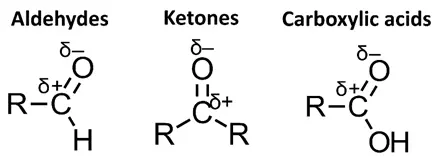
- Carboxylic acids are highly oxidized functional groups, meaning they contain a significant number of bonds to oxygen.
- To reduce a carboxylic acid to a primary alcohol, we need to remove oxygen atoms and add hydrogen atoms.
- This process occurs in two steps: first, the carboxylic acid is reduced to an aldehyde, and then the aldehyde is further reduced to a primary alcohol.
Step 1: Reduction of Carboxylic Acid to Aldehyde
- The reduction of a carboxylic acid to an aldehyde is challenging because carboxylic acids are relatively stable.
- This step typically requires a strong reducing agent, such as lithium aluminum hydride (LiAlH₄).
- The reaction can be summarized as: $$ RCOOH + [H] \rightarrow RCHO + H_2O $$
- Here, $[H]$ represents the reducing agent, which donates hydrogen atoms to the molecule.
Step 2: Reduction of Aldehyde to Primary Alcohol
- Once the aldehyde is formed, it can be further reduced to a primary alcohol.
- This step is easier because aldehydes are more reactive than carboxylic acids.
- The reaction is: $$ RCHO + [H] \rightarrow RCH_2OH $$
Combining these two steps, the overall reduction of a carboxylic acid to a primary alcohol can be written as:
$$ RCOOH + 4[H] \rightarrow RCH_2OH + H_2O $$
- Ethanoic acid ($CH_3COOH$) is reduced to ethanal ($CH_3CHO$) using $LiAlH_4$.
- Ethanal is further reduced to ethanol ($CH_3CH_2OH$).
- Overall reaction:
$$ CH_3COOH + 4[H] \rightarrow CH_3CH_2OH + H_2O $$
Reduction of Ketones to Secondary Alcohols
- Ketones are less oxidized than carboxylic acids, so their reduction is simpler.
- When a ketone is reduced, it gains hydrogen atoms to form a secondary alcohol.
- This reaction also uses a reducing agent like sodium borohydride (NaBH₄) or lithium aluminum hydride (LiAlH₄).
Reaction Mechanism
The reduction of a ketone to a secondary alcohol can be summarized as:
$$ RCOR' + 2[H] \rightarrow RCH(OH)R' $$
In this reaction:
- The carbonyl group ($C=O$) in the ketone is converted into a hydroxyl group ($-OH$).
- Two hydrogen atoms are added: one to the carbon atom and one to the oxygen atom.
- Propanone ($CH_3COCH_3$) is reduced using $NaBH_4$ or $LiAlH_4$.
- The product is isopropanol ($CH_3CHOHCH_3$), a secondary alcohol.
- Reaction:
$$ CH_3COCH_3 + 2[H] \rightarrow CH_3CHOHCH_3 $$
- The reduction of a ketone to a secondary alcohol involves the transfer of a hydride ion ($H^-$).
- The hydride ion, typically supplied by a reducing agent like sodium borohydride ($NaBH_4$) or lithium aluminium hydride ($LiAlH_4$), acts as a nucleophile, attacking the electrophilic carbonyl carbon and leading to the formation of a secondary alcohol.