Practice Endothermic and exothermic reactions with authentic MYP MYP Chemistry exam questions for both SL and HL students. This question bank mirrors Paper 1, 2, 3 structure, covering key topics like atomic structure, chemical reactions, and organic chemistry. Get instant solutions, detailed explanations, and build exam confidence with questions in the style of MYP examiners.
True or False: The products of an endothermic reaction are generally more energetically stable than the reactants because they have absorbed energy.
Enthalpy change () represents the heat energy change of a system measured at constant __________.
In an endothermic reaction, which statement correctly describes the bond energy balance?
In the context of chemical thermodynamics, which statement correctly identifies the "system" and the "surroundings" for a reaction occurring in an aqueous solution in a beaker?
For a specific reaction, the enthalpy of the reactants is and the enthalpy of the products is . How is this reaction classified?
True or False: In an exothermic reaction, the chemical system contains less enthalpy after the reaction than it did before.
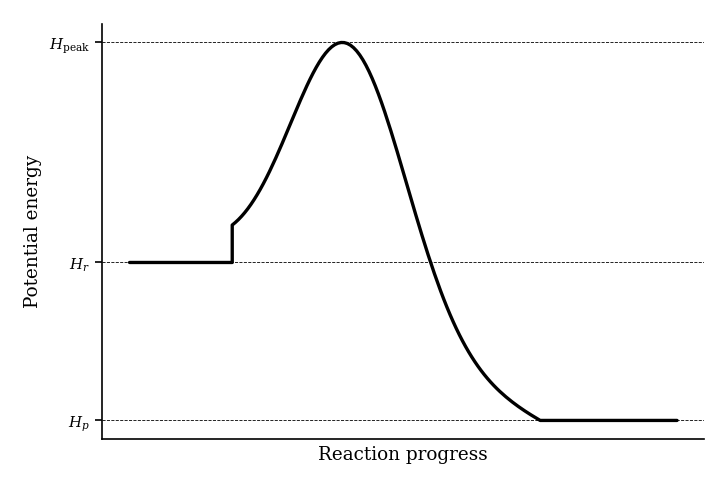
Which mathematical expression correctly calculates the activation energy () for the forward reaction?
Which of the following correctly describes how the activation energy () is measured on an energy profile diagram for an exothermic reaction?
A student dissolves a sample of ammonium nitrate in a beaker of water and observes that the outside of the beaker feels significantly colder. Which set of descriptors correctly identifies this process?
Which statement correctly describes the relationship defined by enthalpy change ()?