S3.1.7 Discontinuities in Ionization Energy Trends (Higher Level Only)
Explanation of Discontinuities in Ionization Energy Trends
General Trend Across a Period
- As you move across a period in the periodic table:
- Nuclear charge increases (more protons in the nucleus).
- Electrons are added to the same principal energy level, so shielding remains relatively constant.
- The increased attraction between the nucleus and the outermost electrons results in a higher ionization energy.
- However, this smooth increase is interrupted at specific points due to the stability of half-filled and fully filled sublevels.
Stability of Half-Filled and Fully Filled Sublevels
- Electrons in an atom are arranged in sublevels (s, p, d, etc.), which have distinct energy levels.
- The stability of an atom's electron configuration depends on how these sublevels are filled.
- Two configurations are particularly stable:
- Half-filled sublevels: Sublevels where each orbital contains one electron (e.g., p³ or d⁵).
- Fully filled sublevels: Sublevels where all orbitals are completely filled (e.g., p⁶ or d¹⁰).
- This stability arises from:
- Symmetry: Half-filled and fully filled sublevels have symmetrical electron distributions, which lower the energy of the atom.
- Exchange energy: Electrons in half-filled sublevels can exchange positions within orbitals, which increases stability due to reduced electron repulsion.
Key Discontinuities in Ionization Energy
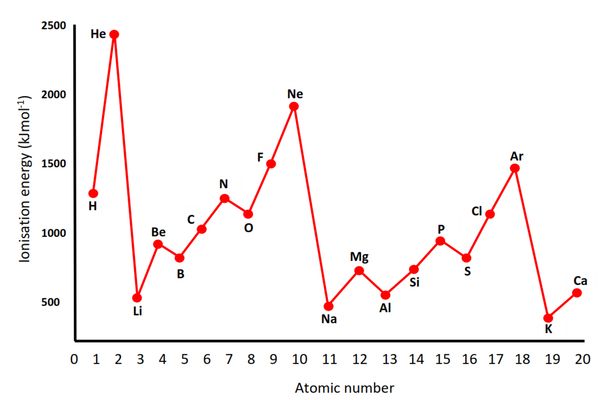
1. Between Group 2 and Group 13 Elements
- Example: $Be$ (Group 2) vs. $B$ (Group 13)
- Observation: The ionization energy of $B$ is lower than expected, despite the general trend of increasing ionization energy across a period.
- Explanation:
- $Be$ has a fully filled 2s sublevel (2s²), which is particularly stable.
- $B$ has an electron in the 2p sublevel (2s² 2p¹).
- The 2p electron is higher in energy and experiences less nuclear attraction due to shielding by the 2s electrons.
- This makes it easier to remove, resulting in a lower ionization energy.
2. Between Group 15 and Group 16 Elements
- Example: $N$ (Group 15) vs. $O$ (Group 16)
- Observation: The ionization energy of $O$ is lower than expected.
- Explanation:
- $N$ has a half-filled 2p sublevel (2p³), which is particularly stable.
- $O$ has one more electron (2p⁴), which introduces electron-electron repulsion within the 2p orbital.
- This repulsion makes it easier to remove an electron from $O$, resulting in a lower ionization energy.
Discontinuities in ionization energy trends are directly linked to the stability of half-filled and fully filled sublevels.
Evidence for Sublevels: Ionization Energy Trends
- The periodic trends in ionization energy provide strong evidence for the existence of sublevels (s, p, and d).
- If electrons were arranged in a simple, uniform manner, ionization energy would increase smoothly across a period.
- However, the observed discontinuities reveal the presence of distinct sublevels with varying stability.
How Ionization Energy Trends Support Sublevels
- Sharp Increases Between Energy Levels
- A significant jump in ionization energy occurs when removing an electron from a lower principal energy level (e.g., from 2p to 1s).
- This indicates that electrons are organized into distinct energy levels and sublevels.
- Discontinuities Within a Period
- The deviations in ionization energy trends (e.g., between Groups 2 and 13 or Groups 15 and 16) align with the filling of sublevels (s, p, d).
- This supports the idea that sublevels have different energy levels and stability.
- Transition Metals and d Sublevels
- The relatively small changes in ionization energy across the transition metals (d-block) reflect the filling of the d sublevel, which is closer in energy to the s sublevel.
- This provides further evidence for the existence of d sublevels.
Evidence from Successive Ionization Energies
- Consider the successive ionization energies of magnesium (Mg):
- 1st ionization energy: 738 $\mathrm{kJ mol}^{-1}$ (removal of 3s¹ electron)
- 2nd ionization energy: 1451 $\mathrm{kJ mol}^{-1}$ (removal of 3s² electron)
- 3rd ionization energy: 7732 $\mathrm{kJ mol}^{-1}$ (removal of 2p⁶ electron)
- The sharp increase between the 2nd and 3rd ionization energies indicates that the 3rd electron is being removed from a lower energy level (2p), providing evidence for distinct sublevels.
- Students often assume that ionization energy always increases smoothly across a period.
- Remember to account for the stability of half-filled and fully filled sublevels when explaining discontinuities.
- Why is the ionization energy of nitrogen higher than oxygen, despite being earlier in the period?
- How do successive ionization energies provide evidence for sublevels?