Gibbs Free Energy, Equilibrium, and Spontaneity
$ΔG$ and Reaction Progress
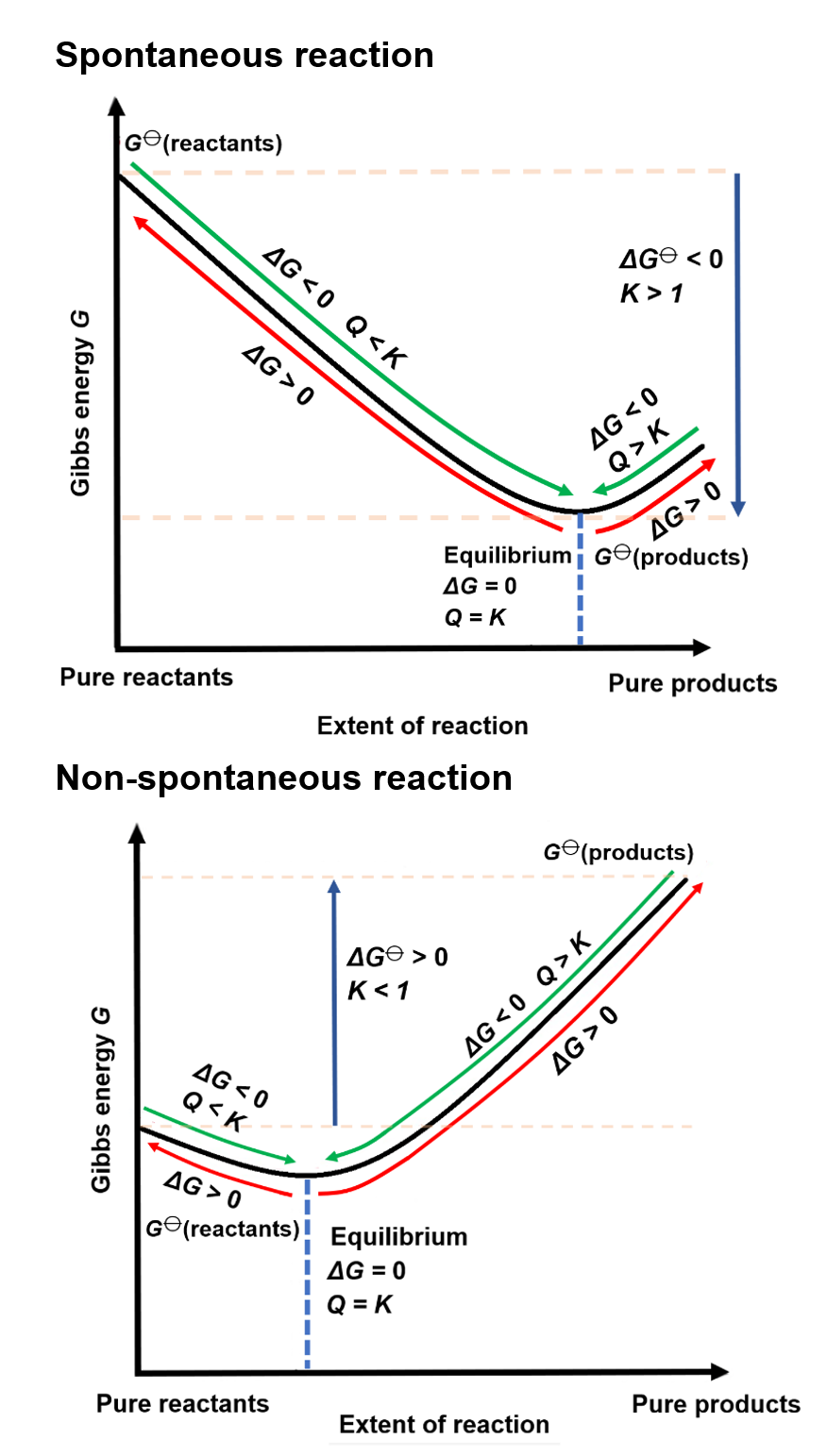
- As discussed in the previous sections, Gibbs free energy ($ \Delta G $) is a thermodynamic quantity that helps us predict whether a reaction is spontaneous under constant pressure and temperature.
- A reaction is spontaneous if $ \Delta G $ is negative, meaning it can proceed without external energy input.
- However, as a reaction progresses, the concentrations of reactants and products change, and so does $ \Delta G $.
- At the start of a reaction, $ \Delta G $ is often negative, favoring the forward reaction.
- As the reaction progresses, $ \Delta G $ becomes less negative as the system approaches equilibrium.
- At equilibrium, $ \Delta G = 0 $. At this point, there is no net change in the concentrations of reactants and products because the forward and reverse reactions occur at the same rate.
- Consider the Haber process: $$ \text{N}_2(g) + 3\text{H}_2(g) \leftrightharpoons 2\text{NH}_3(g) $$
- Initially, $ \Delta G $ is negative, so the formation of ammonia ($ \text{NH}_3 $) is spontaneous.
- As more ammonia is produced, $ \Delta G $ becomes less negative until equilibrium is reached, at which point $ \Delta G = 0 $.
At equilibrium, the system’s Gibbs free energy is at its minimum, representing a state of maximum stability.
The Relationship Between ΔG and the Equilibrium Constant (K)
- At equilibrium, the concentrations of products and reactants form a constant ratio, defined as the equilibrium constant ($ K $).
- The relationship between $ \Delta G $ and $ K $ is expressed by the equation: $$ \Delta G^\circ = -RT \ln K $$ where:
- $ \Delta G^\circ $: Standard Gibbs free energy change ($kJ \, mol^{-1}$) under standard conditions.
- $ R $: Gas constant ($ 8.31 \, \text{J mol}^{-1} \, \text{K}^{-1} $).
- $ T $: Temperature in Kelvin (K).
- $ K $: Equilibrium constant (unitless).
Equilibrium constant $K$ will covered in Reactivity 2.3.
This equation links the thermodynamic favorability of a reaction (as indicated by $ \Delta G^\circ $) to the position of equilibrium (as characterized by $ K $).
Key Insights:
- If $ \Delta G^\circ < 0$:
- The reaction favors products at equilibrium ($ K > 0$)
- If $ \Delta G^\circ > 0 $:
- The reaction favors reactants at equilibrium ($ K <0$)
- If $\Delta G^\circ = 0 $:
- Neither reactants nor products are favored ($ K = 1 $).
Combustion reactions, which are highly exothermic, typically have large $ K $ values.
The decomposition of water into hydrogen and oxygen gases under standard conditions has a very small $ K $ value.
- Students often confuse $ \Delta G $ (which changes as a reaction progresses) with $ \Delta G^\circ $ (which is constant for a reaction under standard conditions).
- Remember that $ \Delta G^\circ $ applies to standard conditions, while $ \Delta G $ depends on the actual concentrations of reactants and products.
Predicting Equilibrium Composition
The sign and magnitude of $ \Delta G^\circ $ provide valuable insights into the equilibrium composition of a reaction mixture:
- If $ \Delta G^\circ <0$:
- The products are more stable than the reactants, so the equilibrium mixture will be product-rich.
- If $\Delta G^\circ > 0$:
- The reactants are more stable than the products, so the equilibrium mixture will be reactant-rich.
For the reaction:
$$ \text{H}_2(g) + \text{I}_2(g) \leftrightharpoons 2\text{HI}(g) $$
Suppose $ \Delta G^\circ = -10 \, \text{kJ mol}^{-1} $.
Since $ \Delta G^\circ< 0 $, the equilibrium constant $ K $ will be greater than 1, indicating that the reaction favors the formation of $ \text{HI} $.
If $ \Delta G^\circ > 0 $, what can you conclude about the equilibrium constant $ K $?
The Relationship Between $ΔG$ and the Reaction Quotient ($Q$)
Reaction quotient
The reaction quotient ($Q$) represents the ratio of product concentrations to reactant concentrations at any point during the reaction:
$$Q = \frac{[\text{products}]}{[\text{reactants}]}$$
- The equation:
- $$\Delta G = \Delta G^\circ + RT \ln Q$$ relates the Gibbs free energy change ($\Delta G$) of a reaction under non-standard conditions to its standard Gibbs free energy change ($\Delta G^\circ$) and the reaction quotient ($Q$).
- When $Q$ differs from the equilibrium constant ($K$), the system is not at equilibrium, and the sign of $\Delta G$ determines whether the reaction will shift towards products or reactants to reach equilibrium.
Reaction quotient will be covered in Reactivity 2.3.
Key Insights from the Equation:
- If $Q < K$: $\Delta G < 0$, the reaction is spontaneous in the forward direction as the system moves toward equilibrium.
- If $Q > K$: $\Delta G > 0$, the reaction is non-spontaneous in the forward direction and will shift towards reactants.
- If $Q = K$: $\Delta G = 0$, the system is at equilibrium with no net change in reactant or product concentrations.
This expression highlights how Gibbs free energy continuously adjusts based on the reaction's progress, emphasizing the dynamic nature of chemical equilibrium.
Calculating $ K $ from $ \Delta G^\circ $
The standard Gibbs free energy change for the reaction $ \text{N}_2(g) + 3\text{H}_2(g) \leftrightharpoons 2\text{NH}_3(g) $ is $ \Delta G^\circ = -33.0 \, \text{kJ mol}^{-1} $ at 298 K. Calculate the equilibrium constant $ K $.
Solution
- Convert $ \Delta G^\circ $ to joules:
$ \Delta G^\circ = -33.0 \, \text{kJ mol}^{-1} = -33,000 \, \text{J mol}^{-1} $ - Use the equation $ \Delta G^\circ = -RT \ln K $:
$ -33000 = -(8.31)(298) \ln K $ - Solve for $ \ln K $:
$ \ln K = \frac{-33000}{-(8.31)(298)} = 13.3 $ - Exponentiate to find $ K $:
$ K = e^{13.3} \approx 6.0 \times 10^5 $ - Interpretation: The large $ K $ value indicates that the reaction strongly favors the formation of ammonia at equilibrium.
Calculating $ \Delta G $ During a Reaction
For the same reaction, if the reaction quotient $ Q = 1.0 \times 10^{-3} $, calculate $ \Delta G $ at 298 K.
Solution
- Use the equation $ \Delta G = \Delta G^\circ + RT \ln Q $:
$ \Delta G = -33000 + (8.31)(298) \ln (1.0 \times 10^{-3}) $ - Calculate $ \ln Q $:
$ \ln (1.0 \times 10^{-3}) = -6.91 $ - Substitute values:
$ \Delta G = -33000 + (8.31)(298)(-6.91) $ - Solve:
$ \Delta G = -33000 - 17150 = -50150 \, \text{J mol}^{-1} $ - Interpretation: Since $ \Delta G< 0 $, the forward reaction is spontaneous under these conditions.
- Why does $ \Delta G $ equal zero at equilibrium?
- How does temperature affect the relationship between $ \Delta G^\circ $ and $ K $?
- For a reaction where $ \Delta G^\circ > 0 $, is it possible to make the forward reaction spontaneous by changing the temperature? Why or why not?