Energy Transfer in Chemical Reactions: Heat vs. Temperature
Energy Transfer in Chemical Reactions
The System and the Surroundings
In every chemical reaction, energy flows between the system (the reactants and products) and the surroundings (everything else, such as the reaction container and the air).
System
The system is the specific part of the universe under study (e.g., the reactants and products involved in the reaction).
Surroundings
Surroundings are everything outside the system that interacts with it (e.g., the reaction flask, water bath, or air).
- The total energy of the universe (system + surroundings) remains constant, as dictated by the law of conservation of energy.
- However, energy can transfer between the system and surroundings in various forms, such as heat, light, or mechanical work.
Types of Systems
To understand how energy is transferred, it’s helpful to categorize systems based on their interaction with the surroundings:
- Open system: Both matter and energy can cross the system's boundaries (e.g., boiling water in an uncovered pot).
- Closed system: Only energy can cross the boundaries, not matter (e.g., a sealed reaction flask).
- Isolated system: Neither matter nor energy can cross the boundaries (e.g., an ideal thermos bottle).
Heat vs. Temperature: What’s the Difference?
While heat and temperature are closely related, they are distinct concepts in thermodynamics.
Heat $q$: Energy in Transfer
Heat
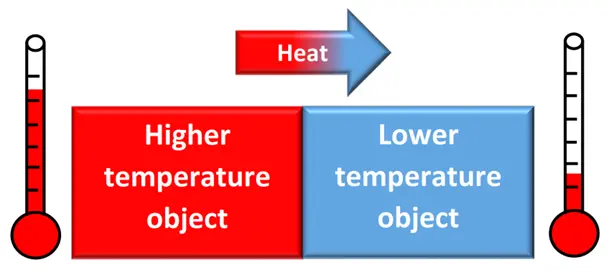
Heat is the energy transferred between a system and its surroundings due to a temperature difference.
- It flows from a warmer object to a cooler one until thermal equilibrium is reached.
- Heat is measured in joules (J) or kilojoules (kJ).
- Heat is not a property of a system: it exists only during energy transfer.
- Heat transfer can cause temperature changes or phase changes (e.g., melting ice).
Temperature $T$: A Measure of Kinetic Energy
Temperature
Temperature measures the average kinetic energy of the particles in a system.
It indicates how "hot" or "cold" a substance is and is measured in degrees Celsius (°C) or kelvin (K).
Unlike heat, temperature is a state function, meaning it depends only on the current state of the system, not how the system reached that state.
- What is the difference between heat and temperature?
- If the temperature of a system increases, does it always mean heat has been added? Why or why not?
- Can heat flow from a colder object to a hotter one? Explain.