Practice Haloalkanes and Haloarenes with authentic CBSE Chemistry exam questions for both SL and HL students. This question bank mirrors Paper 1, 2, 3 structure, covering key topics like atomic structure, chemical reactions, and organic chemistry. Get instant solutions, detailed explanations, and build exam confidence with questions in the style of CBSE examiners.
Write the product(s) formed when:
2-Bromopropane undergoes dehydrohalogenation reaction.
Chlorobenzene undergoes nitration reaction.
Methylbromide is treated with KCN.
Out of and which is more reactive towards SN1 reaction and why?
Which would undergo SN1 reaction faster in the following pair? CH3-CH2-CH2-Br and .
Answer any 3 of the following:
Which isomer of C5H10 gives a single monochloro compound C5H9Cl in bright sunlight?
Arrange the following compounds in increasing order of reactivity towards SN2 reaction: 2-Bromopentane, 1-Bromopentane, 2-Bromo-2-methylbutane
Why p-dichlorobenzene has higher melting point than those of ortho-and meta-isomers?
Identify A and B in the following: 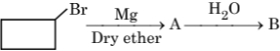
Complete the following reaction equations:
In the following pairs of halogen compounds, which would undergo SN2 reaction faster?
Explain why:
the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
alkyl halides, though polar, are immiscible with water?
Grignard reagents should be prepared under anhydrous conditions?
Write the chemical equations when:
methyl chloride is treated with AgNO2.
bromobenzene is treated with CH3Cl in the presence of anhydrous AlCl3.
Why is boiling point of o-dichlorobenzene higher than p-dichlorobenzene?
Why is melting point of para isomer higher than ortho isomer?
Write the IUPAC name of the following compound:
)
)