Practice R1.3 Energy from fuels with authentic IB Chemistry exam questions for both SL and HL students. This question bank mirrors Paper 1A, 1B, 2 structure, covering key topics like atomic structure, chemical reactions, and organic chemistry. Get instant solutions, detailed explanations, and build exam confidence with questions in the style of IB examiners.
What is the primary chemical process involved when a fuel is used to release energy?
Which of the following fuel sources is considered carbon neutral only if replanted or recycled properly?
What is the definition of fuel in chemistry?
A diagram of a hydrogen fuel cell is shown.
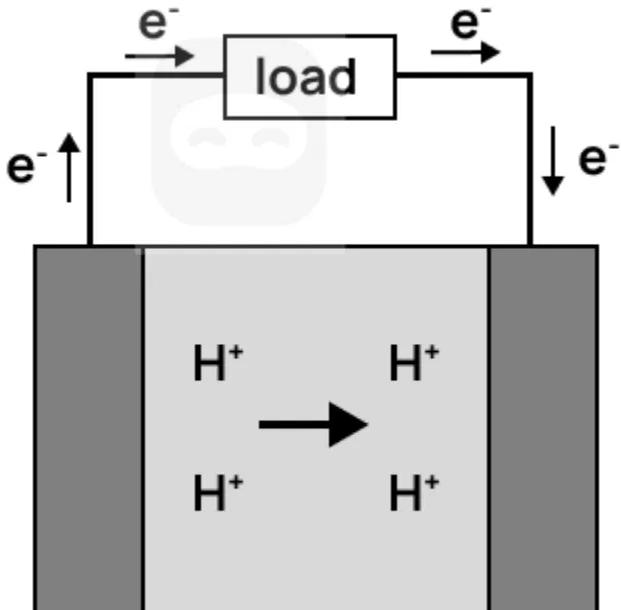
Which statements are correct when the cell operates?
I. Hydrogen gas is oxidised at the anode
II. Pure water is produced at the cathode
III. The flow of electrons is from cathode to anode
Which of the following is a renewable energy source?
A candle is burned in a closed jar with limited oxygen. Over time, black soot forms on the glass, and the flame goes out.
Define incomplete combustion.
Write a balanced chemical equation for the incomplete combustion of methane forming carbon monoxide.
State another possible product of incomplete combustion besides carbon monoxide.
Suggest why incomplete combustion is dangerous in enclosed spaces.
Compare the energy released in complete and incomplete combustion of the same fuel.
Which expression gives the mass, in g, of ethanol required to produce of heat upon complete combustion?
()
Which of the following statements about the combustion of fuels is correct?
Which of the following is not a product of complete combustion of a hydrocarbon fuel?
Which equation represents the complete combustion of ethanol ()?